Abstract
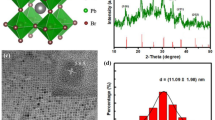
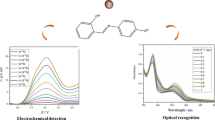
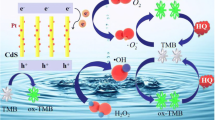
In this study, the halogen exchange reactions are investigated between KI or methylamine iodide in aqueous solution and CsPbBr3 NCs in toluene. The mass transfer process on the interface between water and toluene affects the halogen exchange time and efficiency. Stirring and heating can effectively improve the halogen exchange efficiency and increases the sensing sensitivity. The photoluminescence wavelength shift of CsPbBr3 NCs shows good linear relationship with the concentration of I− in the range from 0 to 20 nmol/L with the detection limit of 0.2 nmol/L I−. Taking H2O2 as a typical water-soluble oxide, the method is applied to the colorimetric sensing of H2O2 in water solution. After the optimization of sensing conditions, the obvious wavelength shift could be observed with the different concentration range of H2O2. A good linear relationship between the wavelength shift and the H2O2 concentration from 0 to 1.0 mmol/L with the detection limit of 0.05 mmol/L H2O2 could be found.





Similar content being viewed by others
Data availability statement
Data are contained within the article and supplementary material.
References
Nedelcu G, Protesescu L, Yakunin S, Bodnarchuk MI, Grotevent MJ, Kovalenko MV. Fast Anion-exchange in highly luminescent nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015;15(8):5635–40.
Akkerman QA, D’Innocenzo V, Accornero S, Scarpellini A, Petrozza A, Prato M, Manna L. Tuning the optical properties of cesium lead halide perovskite nanocrystals by anion exchange reactions. J Am Chem Soc. 2015;137(32):10276–81.
Huang G, Huang Y, Wei Xu, Yao Q, Liu X, Ding C, Chen Xi. Cesium lead halide perovskite nanocrystals for ultraviolet and blue light blockinglight blocking. Chem Mater. 2019;30(5):1021–3.
Uddin MA, Glover JD, Park SM, Pham JT, Graham KR. Growth of highly stable and luminescent CsPbX3 (X = Cl, Br, and I) nanoplates via ligand mediated anion exchange of CsPbCl3 nanocubes with AlX3. Chem Mater. 2020;32(12):5217–25.
Parobek D, Dong Y, Qiao T, Rossi D, Son DH. Photoinduced anion exchange in cesium lead halide perovskite nanocrystals. J Am Chem. 2017;139(12):4358–61.
Doane TL, Ryan KL, Pathade L, Cruz KJ, Zang HD, Cotlet M, Maye MM. Using perovskite nanoparticles as halide reservoirs in catalysis and as spectrochemical probes of ions in solution. ACS Nano. 2016;10(6):5864–72.
Huang Y, Feng Y, Li F, Lin F, Wang Y, Chen Xi, Xie R. Sensing studies and applications based on metal halide perovskite materials: current advances and future perspectives. Trends Anal Chem. 2021;134: 116127.
Zhu Y, Li F, Huang Y, Lin F, Chen Xi. Wavelength-shift-based colorimetric sensing for peroxide number of edible oil using CsPbBr3 perovskite nanocrystals. Anal Chem. 2019;91(22):14183–7.
Guhrenz C, Benad A, Ziegler C, Haubold D, Gaponik N, Eychmuller A. Solid-state anion exchange reactions for color tuning of CsPbX3 perovskite nanocrystals. Chem Mater. 2019;91(22):14183–7.
Yan AP, Guo YL, Liu C, Deng Z, Guo Y, Zhao XJ. Tuning the optical properties of CsPbBr3 nanocrystals by anion exchange reactions with CsX aqueous solution. Nanoscale Res Lett. 2018;185:3517.
Liu HW, Liu ZY, Xu WZ, Yang LT, Liu Y, Yao D, Zhang DQ, Zhang H, Yang B. Engineering the photoluminescence of CsPbX3 (X = Cl, Br, and I) perovskite nanocrystals across the full visible spectra with the interval of 1 nm. ACS Appl Mater. 2019;11(15):14256–65.
Begum R, Chin XY, Damodaran B, Hooper TJN, Mhaisalkar S, Mathews N. Cesium lead halide perovskite nanocrystals prepared by anion exchange for light-emitting diodes. ACS Appl Nano Mater. 2020;3(2):1766–74.
Miller WL, Kester DR. Hydrogen peroxide measurement in seawater by (p-hydroxyphenyl)acetic acid dimerization. Anal Chem. 1988;60(24):2711–5.
Protesescu L, Yakunin S, Bodnarchuk MI, Krieg F, Caputo R, Hendon CH, Yang RX, Walsh A, Kovalenko MV. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015;15(6):3692–6.
Huang SQ, Li ZC, Kong L, Zhu NW, Shan AD, Li L. Enhancing the stability of CH3NH3PbBr3 quantum dots by embedding in silica spheres derived from tetramethyl orthosilicate in “Waterless” toluene. J Am Chem. 2016;138(18):5749–52.
Liu Y, Li FS, Li QQ, Yang KY, Guo TL, Li XM, Zeng HB. Emissions at perovskite quantum dot/film interface with halide anion exchange. ACS Photon. 2018;5(11):4504–12.
Funding
This research was funded by National Natural Science Foundations of China (21876141) and the Shenzhen Science and Technology Project (JCYJ20180306172823786).
Author information
Authors and Affiliations
Contributions
Experiments, investigation, and original draft preparation, H-GL, Y-MZ; methodology, X-LL, Z-YG; review and editing, Y-NH; funding acquisition supervision, and writing, XC. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Li, H., Zhu, Y., Liu, X. et al. Colorimetric Sensing of Hydrogen Peroxide Based on the Wavelength-Shift of CsPbBr3 Perovskite Nanocrystals on Water–Oil Interface. J. Anal. Test. 7, 1–7 (2023). https://doi.org/10.1007/s41664-022-00231-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41664-022-00231-1