Abstract
9-Oxo-10,11-dehydroageraphorone (ODA) is a cadinene sesquiterpene isolated from Eupatorium adenophorum. The antimicrobial activity of ODA was tested in vitro against four fungal strains and four bacterial strains. The fungitoxicity of ODA was evaluated by percentage radial growth inhibition using the poisoned food technique. ODA inhibited Fusarium oxysporum more than the other tested strains, giving EC50 values of 0.325 mg ml−1 at 48 h and 0.365 mg ml−1 at 96 h. Tests also revealed that ODA inhibits the germination of Fusarium oxysporum spores, reducing germination by 97 % at 0.5 mg ml−1 concentration. The minimum inhibitory concentrations (MIC) of ODA against four tested strains of Ralstonia solanacearum ranged from 0.25 to 1 mg ml−1 using a micro-well dilution method. In addition, our scanning electron microscopy study revealed previously unreported, pronounced effects of ODA on Fusarium oxysporum, Bipolaris sorokiniana, Fusarium proliferatum and Alternaria tenuissima, including marked shrinkage in the mycelia of all tested strains, swelling at the ends of Bipolaris sorokiniana mycelia and inhibition of spore production in the strains of Bipolaris sorokiniana and Fusarium proliferatum, providing firm evidence of the toxicity of ODA to these pathogenic fungi.
Similar content being viewed by others
Introduction
Plants produce a wide range of antimicrobial substances. Many plant secondary metabolites have been isolated, such as alkaloids, flavonoids, proteins, organic acids and cadinene sesquiterpenes [30]. Most of the secondary metabolites are effective against pathogenic diseases. These antimicrobial agents preserve plants from pathogens and have been accumulated through long-term evolution [19]. Compared with synthetic chemicals, phytochemicals are of natural origin, safer for human health, biodegradable and generally leave negligible toxic residues.
Eupatorium adenophorum Spreng (Asteraceae) is regarded as a noxious weed worldwide and is distributed in a dozen or more countries [14, 25]. The plant was introduced in China in the 1940s [25] and is mainly distributed in the Yunnan–Guizhou plateau [17]. E. adenophorum is recognized for its abundance of bioactive substances and toxicity to livestock such as horses, cattle and goats [20, 21]. Previous studies have shown that the main toxin of E. adenophorum is 9-oxo-10,11-dehydroageraphorone (ODA), a cadinene sesquiterpene which exists mainly in leaves and flowers [3, 20, 29]. However, experiments have shown that ODA exhibits low toxicity to mammals; the LD50 of ODA is 1470 mg kg−1 body weight based on oral administration for 14 days [22].
Much time and effort has been expended studying the hepatotoxicity [4], pulmonary toxicity [21, 23] and biological pesticidal activity [13] of crude extracts of E. adenophorum. Although ODA has been identified previously by Bohlmann and Gupta [3], only a few studies have accessed the bioactivity of ODA. Kundu et al. [15] reported that ODA has antioxidant activity, and Proksch et al. [24] reported that ODA exhibits contact toxicity and growth retarding activity against the larvae of a noctuid species. Therefore, our study aimed to evaluate the effects of ODA on plant pathogenic fungi and bacteria. In addition, scanning electron microscopy was used to examine whether changes occurred in the hyphae of tested fungi treated with 1 mg ml−1 ODA.
Materials and methods
Extraction and purification of ODA
Leaves of E. adenophorum were collected from Xichang City, Sichuan Province, southwest China, in May 2014. The plant was identified by Prof. A. C. Cao (Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing). A voucher specimen (No. 20060712) was deposited at the College of Chemistry, Beijing Normal University, Beijing 100875, People’s Republic of China. ODA isolation and analysis were carried out using a method that we have published previously [2]. The purity of ODA was greater than 90 % (determined by high-performance liquid chromatography, HPLC). Figure 1 shows the structure of ODA.
Fungal strains
The fungal strains of Fusarium oxysporum, Bipolaris sorokiniana, Fusarium proliferatum and Alternaria tenuissima used in this study were obtained from the Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing. Fusarium oxysporum and Fusarium proliferatum were isolated from infected soil in cucumber fields, Bipolaris sorokiniana was isolated from wheat, and Alternaria tenuissima was isolated from tomato. These strains were cultured in PDA (potato extract 3 g l−1, glucose 20 g l−1 and agar 15 g l−1) at 28 °C.
Bacterial strains
Four strains of Ralstonia solanacearum (R 1–4) were used in this study. R 1, R 2 and R 3 were originally isolated from ginger in Shandong, Sichuan and Guangdong provinces, respectively. R 4 was isolated from tomato in Guangxi Province. All strains were identified by the Institute of Plant Protection, Chinese Academy of Agricultural Sciences, Beijing. These strains were cultured in NA medium (beef extract 3 g l−1, glucose 10 g l−1, peptone 5 g l−1 and yeast extract powder 0.5 g l−1) at 28 °C.
Antifungal assay
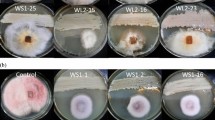
Antifungal assays were carried out using the poisoned food technique [18]. The Petri dishes and medium were autoclaved at 121 °C for 30 min. ODA samples were prepared at different concentrations using dimethyl sulfoxide (DMSO) and diluted by PDA medium at 1.0, 0.8, 0.6, 0.4 and 0.2 mg ml−1. The prepared mixtures were poured into a set of Petri dishes (90 mm diameter) under aseptic conditions. After solidification of the media, a 4-mm mycelial disk was cut from the edge of each 6-day-old culture and placed (with the inoculum facing down) in the center of each PDA plate. Equal amounts of DMSO were added to PDA media to provide the untreated controls. Each treatment was incubated at 28 °C and replicated three times. The mean diameters of fungal colonies were measured with vernier calipers, using a crisscross method, in order to determine the inhibitory rate (I %), as follows [18]:
where C = diameter of fungal colony in the control; T = diameter of fungal colony in the treatment; and 4 = diameter of inoculum disk.
Spore germination inhibition assay
Spores were obtained from 10-day-old fungal cultures. ODA was dissolved in a small amount of DMSO and then diluted with 1 % Tween 80 to obtain the desired concentrations. After combining 0.5 ml ODA stock solution and 0.5 ml spore suspension, 50 µl of the mixed liquid was placed on a glass slide and incubated in a moist chamber at 28 °C until the spore germination rate reached about 90 % in the control group. The same amount of DMSO and 1 % Tween 80 were used as a control, and each test was conducted in triplicate. About 200 spores were counted, and the spore was considered germinated when the length of the germ tube was nearly equal to the short radius of the spore. The spore germination rate (R %) and the spore germination inhibition rate (I %) were calculated using the following equation:
where N g = the number of germinated spores; N t = the number of total observed spores; R 0 = spore germination rate in control group; and R t = spore germination rate in treatment group.
Scanning electron microscopy (SEM) preparation
Samples of the control and the treated fungi were fixed in 2–4 % glutaraldehyde for 4 h at room temperature and then washed in a buffer (0.1 M PBS pH 7.2) for 30 min. The samples were fixed again with 1 % osmium tetroxide for 2 h, washed with redistilled water, dehydrated through a graded series of ethanol (30, 50, 70, 80 and 90 %, for 15–20 min at each step) and then immersed in 100 % acetone twice for 30 min each. The samples were then transferred to a Leica EM CPD030 critical point dryer using liquefied carbon dioxide as transitional fluid. The dried samples were fixed on a sample platform using double-sided adhesive tape. The samples were coated with a thin conductive layer of gold using an Eiko IB5 ion coater and observed in a SEM (FEI Quanta 200F).
Antibacterial assay
The minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) were assessed using a micro-well dilution method [7–9, 16]. The inoculum suspensions were prepared from 24-h broth cultures and adjusted to a 0.5 McFarland standard. Ninety-six-well plates were prepared by dispensing into each well 95 µl of nutrient broth and 5 µl of the inoculum. ODA was dissolved in 5 % DMSO, and then, a twofold broth dilution was used to produce final concentrations of 0.25, 0.5, 1, 2, 4 and 8 mg ml−1 as the stock solutions. Finally, 100 µl stock solution was transferred into six consecutive wells and mixed well by micropipette. The total volume in each well was 200 μl. A well without ODA was used as a negative control. The plates were covered and incubated for 24 h at 28 °C. The MIC was defined as the lowest concentration at which no visual growth of bacteria occurred in any well. The MBC was defined as the lowest concentration at which no visual growth of bacteria occurred on the plates after a period of incubation.
Statistical analysis
All data were analyzed using SPSS 16.0. The median lethal concentration value (EC50) was calculated by the probit model of SPSS 16.0. The spore germination inhibition rate observed at different concentrations of ODA was corrected by eliminating the influence of the control.
Results
Antifungal assay
Our study evaluated the antifungal activity of ODA on four pathogenic fungi using the poisoned food technique. The EC50 values varied from 0.325 to 0.530 mg ml−1 at 48 h and 0.365 to 0.749 mg ml−1 at 96 h (Table 1). Notably, all of the EC50 values increased at 96 h, and this finding may be related to the fungal growth curve [26]. Furthermore, ODA inhibited F. oxysporum more than the other tested organisms, giving EC50 values of 0.325 mg ml−1 at 48 h and 0.365 mg ml−1 at 96 h.
Inhibitory effect of ODA on spore germination
Our study found that ODA has excellent inhibitory activity on spore germination of F. oxysporum, giving 97 % inhibition at 0.5 mg ml−1 concentration, as shown in Table 2. It also exhibited 83 % inhibition of spore germination in B. sorokiniana at 0.5 mg ml−1 concentration, while at the same concentration, only 46 % inhibition of spore germination was observed in F. proliferatum.
Influence of ODA on ultrastructure of fungal pathogens
The SEM results revealed dramatic differences between the control group and the treatment group of four tested fungal strains (Fig. 2). Compared with the control, the mycelia of treated B. sorokiniana, F. proliferatum and A. tenuissima were shrunk substantially, and the ends of B. sorokiniana hyphae swelled notably. Furthermore, ODA induced extensive hyphal branching in B. sorokiniana and some branch points were flattened and irregular. F. oxysporum mycelia developed a small raised papilla at the tip of each branch; the papillae were identified as immature microspores, indicating that ODA delayed the spore production of F. oxysporum. Another important distinction was that the untreated fungal strains, such as B. sorokiniana and F. proliferatum, were able to produce a large number of spores, while the treated group was not able to produce spores.
Antibacterial assay
The antibacterial activity of ODA against R. solanacearum was examined in the present study, and its potency was quantitatively assessed by MIC and MBC values. The results are given in Table 3. A comparison of the bacteriostatic and bactericidal effects of ODA found that the MBC values were higher than the MIC values. ODA at 2 mg ml−1 completely killed all tested strains of R. solanacearum, but the MIC values showed a markedly different pattern: R4 was the most sensitive to ODA with a MIC value of 0.25 mg ml−1, followed by R2, R1 and R3.
Discussion
In previous studies, a crude extract of E. adenophorum was tested against pathogenic fungi [19] and provided a preliminary understanding of its antifungal components. For example, Ahluwalia et al. [1] reported that antifungal oil extracted from E. adenophorum was rich in sesquiterpene, comprising 56 % sesquiterpene in inflorescence oil and 34 % in root oil. Furthermore, five sesquiterpene compounds were isolated from E. adenophorum in 2013, all of which inhibited mycelium growth significantly [14]. Indeed, there are more than 20 different sesquiterpenes in E. adenophorum, most of which have the same molecular skeleton as cadinene (Fig. 1) [12, 14, 22]. ODA belongs to the cadinene sesquiterpene group; terpenes of this type possess three isoprene units and have the empirical formula C15H24. In general, the antimicrobial action of plant essential oils is due to the presence of terpenes [5].
From the perspective of plant protection, this study evaluated the potential role of ODA in crop protection and provided initial findings on the antifungal mechanism of ODA. The resulting EC50 values indicated that ODA exhibited the highest inhibitory activity on F. oxysporum and demonstrated inhibitory activity in the following order: F. oxysporum > F. proliferatum > A. tenuissima > B. sorokiniana (Table 1). ODA also exhibited the greatest inhibitory effect on the spore germination of F. oxysporum, reducing germination by 97 % at 0.5 mg ml−1. Clearly, F. oxysporum is more sensitive to ODA than the other tested pathogens. The different inhibitory effects of ODA on the tested fungal strains may be closely related to the mechanism of ODA action and the physiological structure of the fungi. Our study found that the morphology and ultrastructure of the fungi were severely damaged by ODA.
Diverse antifungal mechanisms have been reported for essential oils, such as leakage of cytoplasmic contents, irreversible damage to cell walls, disruption of cytoplasmic membranes and intracellular organelles, imbalance in intracellular osmotic pressure, reduced total lipid content, delayed conidial production, complete disorganization of hyphal compartments, swelling and deformation of hyphal tips, formation of short branches and collapse of entire hyphae [10, 11, 27]. However, we know that essential oils of plants contain diverse secondary metabolites. Previously, it has been difficult to distinguish the main antifungal compounds. Our present study indicated that ODA may be the main antifungal agent in the essential oil of E. adenophorum. It inhibits hyphae growth and also eliminates the ability of some fungi to produce spores. These findings provide a useful foundation for improving the understanding of the mechanism of ODA against fungi in further studies.
R. solanacearum is the causal agent of bacterial wilt and has been recorded on several hundred plant species distributed in more than 50 families [28]. In China, this disease seriously affects the production of many economically important food crops, such as potato, tomato, ginger and banana. Our study has provided the first data demonstrating that ODA possesses potential activity against R. solanacearum and is especially effective on a strain isolated from tomato (R 4). Although the MIC values differed significantly among the tested strains, four strains of R. solanacearum were completely killed at the concentration of 2 mg ml−1. Moreover, R. solanacearum possesses typical characteristics of a gram-negative bacterium; for example, the cell wall is loose and rich in lipids, while ODA is highly lipophilic and has a low molecular weight. It is therefore possible that ODA may diffuse across cell membranes to induce biological reactions in R. solanacearum and organism with similar characteristics. Although the mechanism of action of ODA has not been verified, terpene’s mode action is speculated to involve membrane disruption by lipophilic compounds [6].
Conclusion
Antifungal and acaricidal activities of ODA have been documented in previous studies [14]. However, studies have not previously demonstrated ODA’s effects on the morphology and ultrastructure of fungi as well as bacteria. The results of this study indicate that ODA has a substantial inhibitory effect on both hyphal growth and spore germination and even affects the reproductive growth of mycelia. It also clearly inhibits R. solanacearum strains. Since ODA possesses antimicrobial properties and can be easily extracted from E. adenophorum, it can be used as a natural antimicrobial agent in new agrochemicals for suppressing plant disease.
References
Ahluwalia V, Sisodia R, Walia S, Sati OP, Kumar J, Kundu A (2014) Chemical analysis of essential oils of Eupatorium adenophorum and their antimicrobial, antioxidant and phytotoxic properties. J Pest Sci 87:341–349
Bai J, Cao AC, Guo MX, Liu XY, Liu XW, Liang H, Zhou B (2011) Identification of 9-oxo-10,11-dehydroagerophorone in Eupatorium adenophorum by high performance liquid chromatography. Chin Bull Bot 46:470–475
Bohlmann F, Gupta R (1981) Six cadinene derivatives from Ageratina adenophora. Phytochemistry 20:1432–1433
Bhardwaj R, Singh A, Sharma OP, Dawra RK, Kurade NP, Mahato SB (2001) Hepatotoxicity and cholestasis in rats induced by the sesquiterpene, 9-oxo-10,11-dehydroageraphorone, isolated from Eupatorium adenophorum. J Biochem Mol Toxic 15:279–286
Cardile V, Russo A, Formisano C, Rigano D, Senatore F, Arnold NA, Piozzi F (2009) Essential oils of Salvia bracteata and Salvia rubifolia from Lebanon: chemical composition, antimicrobial activity and inhibitory effect on human melanoma cells. J Ethnopharmacol 126:265–272
Cowan MM (1999) Plant products as antimicrobial agents. Clin Microbiol Rev 12:564–582
Eloff JN (1998) A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med 64:711–713
Gulluce M, Adiguzel A, Ogutcu H, Sengul M, Sahin F (2004) Antimicrobial effects of Quercus ilex L. extract. Phytother Res 18:208–211
Gulluce M, Sahin F, Sokmen M, Ozer H, Daferera D, Sokmen A, Polissiou M, Adiguzel A, Ozkan H (2007) Antimicrobial and antioxidant properties of the essential oils and methanol extract from Mentha longifolia L. ssp. Longifolia. Food Chem 103:1449–1456
Giordani R, Cardenas ML, Moulin-Traffort J, Regli P (1996) Fungicidal activity of latex sap from Carica papaya and antifungal effect of d (+)-glucosamine on Candida albicans growth. Mycoses 39:103–110
Helal GA, Sarhan MM, Abu Shahla ANK, Abou El-Khair EK (2006) Effects of Cymbopogon citratus L. essential oil on the growth, lipid content and morphogenesis of Aspergillus niger ML2-strain. J Basic Microbiol 46:456–469
He L, Yang J, Cao AC, Liu YM, An Y, Shi JG (2006) A new sequiterpenoid from Eupatorium adenophorum Spreng. Chin J Chem 24:1375–1377
Jin Y, Hou LY, Zhang MZ, Tian ZF, Cao AC, Xie XM (2014) Antiviral activity of Eupatorium adenophorum leaf extract against tobacco mosaic virus. Crop Prot 60:28–33
Kundu A, Saha S, Sureshwalia Shakil NA, Kumar J, Annapurna K (2013) Cadinene sesquiterpenes from Eupatorium adenophorum and their antifungal activity. J Environ Sci Health B 48:516–522
Kundu A, Saha S, Walia S, Ahluwalia V, Kaur C (2013) Antioxidant potential of essential oil and cadinene sesquiterpenes of Eupatorium adenophorum. Toxico Environ Chem 95:127–137
Li Y, Li J, Li Y, Wang XX, Cao AC (2013) Antimicrobial constituents of the leaves of Mikania micrantha H. B. K. Plos One 8:1–10
Lu ZJ, Ma KP (2004) The influence of topographical factors on the invasion of the alien species, Eupatorium adenophorum. Acta Phytoecol Sin 28:761–767
Mu LY (1994) The research methods of plant chemical protection. Chinese Agricultural Press, Beijing
Nabin B, Geeta S (2009) Antibacterial and antifungal effect of Eupatorium adenophorum Spreng against bacterial and fungal isolates. Nepal J Sci Technol 10:91–95
Oelrichs PB, Calanasan CA, Macleod JK, Seawright AA, Ng JC (1995) Isolation of a compound from Eupatorium adenophorum (Spreng.) [Ageratina adenophora (Spreng.)] causing hepatotoxicity in mice. Nat Toxins 3:350–354
O’Sullivan BM (1985) Investigations into Crofton weed (Eupatorium adenophorum) toxicity in horses. Aust Vet J 62:30–32
Ouyang CB, Liu XM, Liu Q, Bai J, Li HY, Li Y, Wang QX, Yan DD, Mao LG, Cao AC, Guo MX (2015) Toxicity assessment of cadinene sesquiterpenes from Eupatorium adenophorum in mice. Nat Prod Bioprospect 5:29–36
O’Sullivan BM (1979) Crofton weed (Eupatorium adenophorum) toxicity in horses. Aust Vet J 55:19–21
Proksch P, Wray V, Isman MB, Rahaus I (1990) Ontogenetic variation of biologically active natural products in Ageratina adenophora. Phytochemistry 29:453–457
Sun XY, Lu ZH, Sang WG (2004) Review on studies of Eupatorium adenophorum—an important invasive species in China. J For Res 15:319–322
Supreetha S, Sharadadevi M, Sequeira PS, Jithesh J, Shreyas T, Amit M (2011) Antifungal activity of ginger extract on Candida albicans: an in vitro Study. J Dent Sci Res 2:1–5
Tripathi A, Sharma N, Sharma V (2009) In vitro efficacy of Hyptis suaveolens L. (Poit.) essential oil on growth and morphogenesis of Fusarium oxysporum f. sp. gladioli (Massey) Snyder & Hansen. World J Microb Biot 25:503–512
Wicker E, Grassart L, Coranson-Beaudu R, Mian D, Guilbaud C, Fegan M, Prior P (2007) Ralstonia solanacearum strains from Martinique (French West Indies) exhibiting a new pathogenic potential. Appl Environ Microb 71:6790–6801
Zhao X, Guo WZ, Xue MN, Wei QL, Fu SW, Sheng HL (2009) Terpenes from Eupatorium adenophorum and their allelopathic effects on Arabidopsis seeds germination. J Agric Food Chem 57:478–482
Zhou LJ, Huang JG, Xu HH, An YX, Zhang XW (2006) Anti-microbial activities and active ingredients of compositae plants. Acta Bot Boreali-Occidentalia Sin 26:1959–1964
Acknowledgments
We thank Dr. Melanie Miller for editing the manuscript. This work was supported by the Special Fund for Agro-scientific Research in the Public Interest of China (No. 20110327).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Xiaoman Liu, Canbin Ouyang, Qiuxia Wang, Yuan Li, Dongdong Yan, Dongsheng Yang, Meixia Guo and Aocheng Cao declare that they have no conflict of interest.
Additional information
Xiaoman Liu and Canbin Ouyang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, X., Ouyang, C., Wang, Q. et al. Evaluation of antibacterial and antifungal properties of 9-oxo-10,11-dehydroageraphorone extracted from Eupatorium adenophorum . J Plant Dis Prot 123, 93–99 (2016). https://doi.org/10.1007/s41348-016-0006-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41348-016-0006-3