Abstract
The first case of Coronavirus Disease 2019 (COVID-19), which is caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), in Europe was officially confirmed in February 2020. On 11 March 2020, after thousands of deaths from this disease had been reported worldwide, the WHO changed their classification of COVID-19 from a public health emergency of international concern to a pandemic. The SARS-CoV-2 virus has been shown to be much more resistant to environmental degradation than other coated viruses. Several studies have shown that environmental conditions can influence its viability and infectivity. This review summarizes current knowledge on the transmission pathways of the novel coronavirus, and directs attention towards potentially underestimated factors that affect its propagation, notably indoor spread and outdoor risk sources. The contributions of significant indoor factors such as ventilation systems to the spread of this virus need to be carefully ascertained. Outdoor risk sources such as aerosolized particles emitted during wastewater treatment and particulate matter (PM), both of which may act as virus carriers, should be examined as well. This study shows the influence of certain underestimated factors on the environmental behavior and survival of the SARS-CoV-2 virus. These aspects of coronavirus propagation need to be accounted for when devising actions to limit not only the current pandemic but also future outbreaks.
Graphic abstract

Similar content being viewed by others
Introduction
Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS)—which are caused by the two coronaviruses SARS-CoV and MERS-CoV, respectively—are responsible for large-scale respiratory disease outbreaks that have led to the deaths of thousands of people (Raoult et al. 2020). SARS was identified in China 18 years ago, while MERS was first discovered 8 years ago in Saudi Arabia (Wang et al. 2020a, b). These viruses are highly pathogenic to humans and are believed to have originated from bats. In December 2019, an outbreak of pneumonia in Wuhan (China) attracted tremendous attention due to the rapid spread of this disease worldwide. The then-unknown cause of the pneumonia (later called COVID-19) has since been identified as the novel coronavirus SARS-CoV-2. This virus shares only 79.6% of its gene sequence with SARS-CoV (Zhou et al. 2020). In 2019, Fan et al. (2019) seemed to predict the pandemic declared by the World Health Organization (WHO) on 11 March 2020, stating that: “It is highly likely that future SARS- or MERS- like coronavirus outbreaks will originate from bats, and there is an increased probability that this will occur in China. Therefore, the investigation of bat coronaviruses becomes an urgent issue for the detection of early warning signs, which in turn minimizes the impact of such future outbreaks in China” (Fan et al. 2019).
The novel coronavirus has infected many more people than MERS and SARS have (Guarner 2020; Velavan and Meyer 2020; Wang et al. 2020a, b). Various factors have contributed to the rapid spread of COVID-19. At the initial phase of the pandemic, these included the high density of transport connections between Wuhan (one of the principal transportation hubs in China) and the rest of the world, increasing the chance of person-to-person interactions. In January 2020, Lai et al. estimated that the mean basic reproduction number (R0) of the virus was between 2 and 3.5 at that time in China; these reproduction numbers represent low and high levels of contagiousness, respectively. Velavan and Meyer (2020) reported that the average incubation time of the virus is ~ 6 days, and that it has three principal symptoms: fever, a dry cough, and shortness of breath. Guarner (2020) highlighted the role of SARS-CoV-2 in the pneumonia outbreak. Several vaccines have since been developed for SARS-CoV-2. These are based on the inactivated virus, RNA, DNA, nonreplicating and replicating viral vectors, and protein subunits (Dong et al. 2020). The most promising of the 180 vaccines that are at various stages of development are NVX-CoV2373 from Novavax, ChAdOx1 nCoV-19 from Oxford/AstraZeneca, and BNT162b1 and BNT162b2 from Pfizer/BioNTech (Krammer 2020). In addition, several drugs and therapeutic treatments have been used with varying success to alleviate the pneumonia caused by the novel coronavirus, including remdesivir (in the USA; Zhu et al. 2020), Arbidol (in Russia; Deng et al. 2020), favipavir (in Japan; Shiraki and Daikoku 2020), hydroxychloroquine (Gautret et al. 2020), and convalescent plasma donated by recovered patients (Shen et al. 2020; Zhang et al. 2020; Liu et al. 2020a). The fatality rates reported by different studies range from 2 to 5% (Jung et al. 2020; Li et al. 2020a; Schröder 2020).
The primary action taken by most governments around the world to contain the virus has been to lock down cities and other localities in which SARS-CoV-2 infections have been recorded. Furthermore, real-time monitoring and identification of infected people have been implemented by public institutions and health authorities to track the spread of the virus. This highlights the notion that the learning process early during an unknown virus outbreak is a crucial influence on the effectiveness of decisions made to tackle the outbreak (Lai et al. 2020; Naddeo 2020).
WHO has suggested that personal protective equipment (PPE) such as a long-sleeved gown, gloves, boots, a mask, and goggles or a face shield should be used for individual protection against SARS-CoV-2. Furthermore, WHO identified the use of a bleach solution (2–10% sodium hypochlorite) as an actionable method of disinfecting urban areas potentially contaminated with SARS-CoV-2.
The various transmission pathways of the novel coronavirus are currently being identified. This review summarizes current knowledge on SARS-CoV-2 transmission pathways and highlights the need to pay more attention to currently underestimated factors, such as indoor spread and outdoor risk sources. It is hoped that the points raised in this review will help governmental institutions and other cognizant authorities in their decision-making during the management of viruses outbreak.
Knowledge of actual dispersion and transmission pathways
WHO has reported that this respiratory disease can be transmitted through respiratory droplets (particle diameter > 5–10 μm) and droplet nuclei (particle diameter < 5 μm) (WHO 2020). Droplet nuclei can remain suspended in the air and travel for long distances depending on the turbulence and flow of the air (WHO 2014). In general, airborne particles of size < 5 μm may remain suspended for hours depending on the air flow rate, turbulence, temperature, and humidity (Seto 2015). As reported by Li et al. (2020b) and Leung et al. (2020), an aerosol with particles of size > 10—20 μm can travel through the air for a short time and for a distance of < 1 m. Zhao et al. (2019) highlighted that aerosol size, air velocity, temperature, humidity, and flow rate are the main variables that significantly influence the dispersion of airborne pathogens.
Coronaviruses with sizes ranging from 0.05 to 0.20 μm are single-stranded RNA viruses with crown-like spike proteins on their surfaces (Wang et al. 2020a, b). These viruses can be aerosolized in droplet nuclei 5–10 μm in size (Leung et al. 2020). They are also non-lipid-membrane viruses, meaning that they are highly stable at high relative humidity (70–90%) (Wu et al. 2020a, b). Temperature can affect the states of viral proteins (including enzymes) and the virus genome. As reported by Pastorino et al. (2020) and Rabenau et al. (2005), temperatures above 60 °C decrease coronavirus infectivity in vitro, and maintaining this temperature for more than 30 min generally leads to SARS-CoV-2 inactivation. The surrounding layer of organic material acts as a moderate shield, protecting the virus from other environmental factors and hence increasing its infectivity for a long period. However, the temperature and humidity of its environment affect the transmissibility of the virus. Wang et al. observed that while high temperature and high humidity can both reduce the effective reproductive number (R value) associated with the spread of the virus, the R value cannot be decreased to less than unity (R < 1)—in which case the epidemic would die out gradually—by applying these measures (high temperature and high humidity) alone (Wang et al. 2020a, b).
Infected patients generate infectious droplets of varying sizes through breathing, coughing, or sneezing (WHO 2020). They also produce fecal matter that is rich in SARS-CoV-2 viruses (Wu et al. 2020a, b). As reported by the ISS (Istituto Superiore della Sanità, Italy), up to 200 droplets with diameters ranging from 1 to 24 μm can be emitted during conversations and breathing; these droplets spread over a distance of ~ 1 m (Bonadonna et al. 2020). This result was determined experimentally by a standardized method that involves measuring the droplets emitted by a person as they count the numbers from 1 to 100 out loud (Duguid 1946). Coughing and sneezing can generate up to 350,000 droplets with diameters ranging from 1 to 1000 μm that spread over a distance of ~ 2–3 m. Figure 1 summarizes these observations and presents other confirmed dispersion and transmission pathways of SARS-CoV-2 that are associated with direct and/or indirect contact.
Many studies have demonstrated that the spread and transmissibility of SARS-CoV-2 are much greater in indoor environments, especially in hospitals, laboratories, and schools, due to the presence of or proximity to viral sources as well as the increased possibility of direct contact with infected people or items (Huang et al. 2020). It was initially believed that coronaviruses cannot survive outdoors until Van Doremalen et al. (2020) found viable viral particles 3 h after they had been aerosolized. Van Doremalen et al. tested the viability of SARS-CoV-2 on different solid surfaces; their results showed that the virus was more stable on plastic and stainless steel than on copper and cardboard. Specifically, viable viruses were detected after 72, 24, and 4 h on plastic and steel, cardboard, and copper surfaces, respectively. Experimental tests were carried out at room temperature (22 °C) and a relative humidity of ~ 40%.
Underestimated indoor spread and boost factors
Indoor environments in universities, schools, prisons, health care facilities, assisted living organizations, and daycare centers are areas where the spread and transmissibility of the novel coronavirus can be really high due to the large number of individuals present, the frequency of close interactions, and the likelihood of touching potentially contaminated items (Dietz et al. 2020). People spend more than 90% of their time in closed environments, so the possibility of being infected in them is potentially high.
Underestimated virus spread and boost factors in indoor environments are often associated with ventilation/filtration air systems. La Rosa et al. (2013) reported that it was possible for the virus to spread between passengers seated > 10 m apart in an airplane.
Park et al. (2020) identified a cluster of COVID-19 cases in a call center located in Seoul (South Korea), where 94 (43.5%) of the workers tested positive for SARS-CoV-2. Virus transmission between these workers likely occurred through direct and indirect contact with infected objects and persons and via short- and long-distance dispersion boosted by the ventilation system.
Lu et al. (2020) examined the transmission of the novel coronavirus in a restaurant without natural air circulation, where the clients were seated > 1 m apart. This study found that the main reason for the transmission of the virus in the artificial air flow was the air conditioner, which enabled the infectious droplets to travel for distances of > 1 m. This was confirmed by the observation that the people seated in the direction of the air stream were infected.
Table 1 summarizes the findings of a number of recent case studies in which heating, ventilation, and air conditioning (HVAC) systems were found to have significant potential to spread SARS-CoV-2 and boost its transmission.
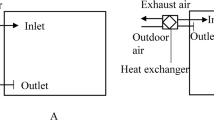
As reported by Bonadonna et al. (2020), depending on the type of HVAC system considered, the distance and the velocity of the spread can lead to three different zones with different probabilities of droplet dragging (Fig. 2). Velocities above 2 m/s will drag droplets, whilst there is a high probability that droplets will be dragged when the velocity is between 1 and 1.9 m/s. There is a low probability that droplets will be dragged when the velocity is between 0.9 and 0.25 m/s.

Adapted from Bonadonna et al. (2020)
Droplet dragging by a HVAC system
To reduce the risk of infection in indoor environments, any HVAC system without an efficient air filtration apparatus should be upgraded. Efficient management (e.g periodically cleaning and sanitation) of the ventilation/filtration system should be done regularly to stop pathogens from persisting on the surface of the filter. Increasing natural ventilation, not lingering directly under the air flow, and minimizing spaces that are shared by people could help to minimize the spread of the virus in indoor environments.
Underestimated outdoor risk sources
The long-distance transmission of viruses in outdoor environments has been confirmed by several studies (Andersen et al. 2009; Zhao et al. 2019). Van Doremalen et al. (2020) found that SARS-CoV-2 can be transmitted via aerosols, as the virus can remain viable and infectious for hours in an aerosol, and for days on surfaces. Andersen et al. (2009) reported that smoke and dust can contribute to the dispersion of viruses over long distances. Setti et al. (2020) found a high correlation (R2 = 0.98) between the number of new coronavirus infections in the north of Italy and the concentration of particulate matter ≤ 10 µm in diameter (PM10) in the air. This relationship would seem to indicate that fine dust particles can act as a carrier for the SARS-Cov-2 virus, boosting its spread and viability. This finding, if confirmed, implies that it is important to consider the involvement of novel outdoor environmental factors in SARS-CoV-2 dissemination, and thus to adopt adequate control actions.
Outdoor environments may lead to a higher risk of infection than indoor environments due to a lack of knowledge regarding the importance of outdoor environments as potential pathways for SARS-CoV-2 infection, the presence of large numbers of people in such environments, and the high likelihood of contact with contaminated elements or items. It is therefore essential to identify any risk factors in outdoor environments in order to prevent the spread of infection. Among these risk factors, wastewater may have an underestimated high potential for SARS-CoV-2 transmission (Naddeo and Liu 2020). Korzeniewska (2011) highlighted how wastewater can be a potential hotspot for the spread of viruses, in particular enteric viruses, because wastewater can provide an excellent environment for the growth of microorganisms. Gundy et al. (2009) proved that a coronavirus can survive in wastewater for periods ranging from a few hours to 2–3 days, depending on the temperature and the chemical composition of the wastewater. Many viruses have been shown to be present in wastewater (Corpuz et al. 2020), including an adenovirus (Kuo et al. 2010), a norovirus (Grøndahl-Rosado et al. 2014), and hepatitis E virus (La Rosa et al. 2010), which can become aerosolized due to their small sizes (< 1 µm). However, the viability of SARS-CoV-2 in wastewater has not been proven, so further studies are needed to investigate its fate in wastewater in detail. Masclaux et al. (2014) found up to 2 × 106 viral particles/m3 in air samples in areas surrounding the treatment units in wastewater treatment plants (WWTPs). Wu et al. (2020a, b) found SARS-CoV-2 viruses in fecal samples, while Casanova et al. (2009) demonstrated that fecally contaminated liquid droplets are a potential vehicle for the spread of this virus. A number of studies have documented the presence of novel coronavirus RNA in wastewater (Ahmed et al. 2020; Alpaslan Kocamemi et al. 2020; Nemudryi et al. 2020; Randazzo et al. 2020; Rosa et al. 2020; Wu et al. 2020a, b; Wurtzer et al. 2020) (Table 2). A computational model developed by Hart and Halden (2020) estimated that the abundance of SARS-COV-2 in wastewater at 20 °C decreased by > 99% over the course of 2–3 days. Chin et al. (2020) demonstrated that SARS-CoV-2 can remain active and detectable in viral transport medium for 14 and 2 days at 22 and 37 °C, respectively. Conversely, Patel et al. (2020) reported that SARS-CoV-2 remains active for 25 days in wastewater at 5 °C. Finally, Bivins et al. (2020) determined that SARS-CoV-2 persisted in wastewater at room temperature for approximately 6 days.
Hospital fecal discharges represent a very important potential source of SARS-Cov-2 transmission, due to the presence of a large number of infected patients. Where sewers are uncovered and open, they could be a major source of SARS-CoV-2 transmission, primarily due to the formation of contaminated aerosolized particles. Specific actions such as covering the sewers and using appropriate disinfection treatments and aerosolized air control systems can be highly effective and should therefore be urgently adopted, especially for these “open” sources, in order to limit or completely suppress their ability to infect and mass contaminate. In this context, it is notable that WWTPs treat their effluents using physical/chemical and/or biological processes (Muñoz et al. 2015; Senatore et al. 2020) but they do not inactivate or disinfect aerosolized virus particles. One possible strategy to minimize the risk posed by aerosolized particles is to implement an air treatment unit (e.g., an ozone generator or UV lamps) that effectively inactivates airborne viruses, including the novel coronavirus. SARS-CoV-2 is vulnerable to solar radiation and is effectively inactivated by UV radiation (Nicastro et al. 2020; Ratnesar-Shumate et al. 2020; Sagripanti and Lytle 2020). UV-B (λ = 280–315 nm) and UV-A (λ = 315–400 nm) radiation are able to penetrate through the Earth’s atmosphere to some extent, and could be an important factor in the fight against the SARS-CoV-2 virus in geographical areas where solar radiation is abundant, such as in the Mediterranean region (Sagripanti and Lytle 2020).
Future perspectives and challenges
HVAC systems, aerosolized particles from wastewater, and particulate matter (PM) have been identified as underestimated infection pathways for the SARS-CoV-2 virus. These pathways can boost and spread COVID-19 transmission in indoor and outdoor environments, so they require careful consideration when attempting to limit the spread of the virus. Specific actions must be implemented by regulatory bodies to account for these risk factors, as they cannot be completely eliminated by social distancing alone. Further research into indoor and outdoor air quality—specifically, into measurement and monitoring strategies for controlling various pathogens—must be conducted to prevent and counter SARS-CoV-2 transmission and other potential biological risks. For the same reason, pathways that allow the virus to spread via solid waste and wastewater sector operations need to be carefully investigated. Infectious PPE waste management is another serious challenge to any governmental and/or other public health agency, given the large volumes of PPE waste that are expected to result from the COVID pandemic. The challenge is therefore to find alternative solutions for managing and disposing of used PPE.
Abbreviations
- COVID-19:
-
Coronavirus Disease 2019
- HVAC:
-
Heating, ventilation, and air conditioning
- ISS:
-
Istituto Superiore della Sanità
- MERS:
-
Middle East Respiratory Syndrome
- PPE:
-
Personal protective equipment
- SARS:
-
Severe Acute Respiratory Syndrome
- SARS-CoV-2:
-
Severe Acute Respiratory Syndrome Coronavirus 2
- WHO:
-
World Health Organization
- WWTP:
-
Wastewater treatment plant
- PM:
-
Particulate matter
References
Ahmed W, Angel N, Edson J, Bibby K, Bivins A, O’Brien JW, Choi PM, Kitajima M, Simpson SL, Li J, Tscharke B, Verhagen R, Smith WJM, Zaugg J, Dierens L, Hugenholtz P, Thomas KV, Mueller JF (2020) First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci Total Environ 728:138764. https://doi.org/10.1016/j.scitotenv.2020.138764
Alpaslan Kocamemi B, Kurt H, Hacioglu S, Yarali C, Saatci AM, Pakdemirli B (2020) First data-set on SARS-CoV-2 detection for Istanbul wastewaters in Turkey. MedRxiv. https://doi.org/10.1101/2020.05.03.20089417
Andersen GL, Frisch AS, Kellogg CA, Levetin E, Lighthart B, Paterno D (2009) Aeromicrobiology/air quality. In: Schaechter M (ed) Encyclopedia of microbiology, 3rd edn. Academic, Boston, pp 11–26. https://doi.org/10.1016/B978-012373944-5.00166-8
Bivins A, Greaves J, Fischer R, Yinda KC, Ahmed W, Kitajima M, Munster VJ, Bibby K (2020) Persistence of SARS-CoV-2 in water and wastewater. Environ Sci Technol Lett. https://doi.org/10.1021/acs.estlett.0c00730
Bonadonna L, La Rosa G, Settimo G, Sorrentino E, Veschetti E, Bertinato L (2020) Indicazioni sugli impianti di ventilazione/climatizzazione in strutture comunitarie non sanitarie e in ambienti domestici in relazione alla diffusione del virus SARS-CoV-2. https://www.iss.it/rapporti-covid-19/-/asset_publisher/btw1J82wtYzH. Accessed July 2020
Casanova L, Rutala WA, Weber DJ, Sobsey MD (2009) Survival of surrogate coronaviruses in water. Water Res 43(7):1893–1898. https://doi.org/10.1016/j.watres.2009.02.002
Chin AWH, Chu JTS, Perera MRA, Hui KPY, Yen H-L, Chan MCW, Peiris M, Poon LLM (2020) Stability of SARS-CoV-2 in different environmental conditions. Lancet Microbe 1(1):e10. https://doi.org/10.1016/s2666-5247(20)30003-3
Corpuz MVA, Buonerba A, Vigliotta G, Zarra T, Ballesteros F, Campiglia P, Belgiorno V, Korshin G, Naddeo V (2020) Viruses in wastewater: occurrence, abundance and detection methods. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2020.140910
Deng L, Li C, Zeng Q, Liu X, Li X, Zhang H, Hong Z, Xia J (2020) Arbidol combined with LPV/r versus LPV/r alone against corona virus disease 2019: a retrospective cohort study. J Infect. https://doi.org/10.1016/j.jinf.2020.03.002
Dietz L, Horve PF, Coil DA, Fretz M, Eisen JA, Van Den Wymelenberg K (2020) 2019 Novel coronavirus (COVID-19) pandemic: built environment considerations to reduce transmission. mSystems. https://doi.org/10.1128/msystems.00245-20
Dong Y, Dai T, Wei Y, Zhang L, Zheng M, Zhou F (2020) A systematic review of SARS-CoV-2 vaccine candidates. Sig Transduct Target Ther. https://doi.org/10.1038/s41392-020-00352-y
Duguid JP (1946) The size and the duration of air-carriage of respiratory droplets and droplet-nuclei. J Hyg. https://doi.org/10.1017/S0022172400019288
Fan Y, Zhao K, Shi ZL, Zhou P (2019) Bat coronaviruses in China. Viruses 11(3):27–32. https://doi.org/10.3390/v11030210
Gautret P, Lagier J-C, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Dupont HT, Honoré S, Colson P, Chabrière E, La Scola B, Rolain J-M, Brouqui P, Raoult D (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. https://doi.org/10.1016/j.ijantimicag.2020.105949
Grøndahl-Rosado RC, Yarovitsyna E, Trettenes E, Myrmel M, Robertson LJ (2014) A one year study on the concentrations of norovirus and enteric adenoviruses in wastewater and a surface drinking water source in Norway. Food Environ Virol. https://doi.org/10.1007/s12560-014-9161-5
Guarner J (2020) Three emerging coronaviruses in two decades. Am J Clin Pathol. https://doi.org/10.1093/ajcp/aqaa029
Gundy PM, Gerba CP, Pepper IL (2009) Survival of coronaviruses in water and wastewater. Food Environ Virol 1(1):10–14. https://doi.org/10.1007/s12560-008-9001-6
Guo Z-D, Wang Z-Y, Zhang S-F, Li X, Li L, Li C, Cui Y, Fu R-B, Dong Y-Z, Chi X-Y, Zhang M-Y, Liu K, Cao C, Liu B, Zhang K, Gao Y-W, Lu B, Chen W (2020) Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis. https://doi.org/10.3201/eid2607.200885
Hart OE, Halden RU (2020) Computational analysis of SARS-CoV-2/COVID-19 surveillance by wastewater-based epidemiology locally and globally: feasibility, economy, opportunities and challenges. Sci Total Environ 730:138875. https://doi.org/10.1016/j.scitotenv.2020.138875
Huang H, Fan C, Li M, Nie HL, Wang FB, Wang H, Wang R, Xia J, Zheng X, Zuo X, Huang J (2020) COVID-19: a call for physical scientists and engineers. ACS Nano. https://doi.org/10.1021/acsnano.0c02618
Jung S-M, Akhmetzhanov AR, Hayashi K, Linton NM, Yang Y, Yuan B, Kobayashi T, Kinoshita R, Nishiura H (2020) Real-time estimation of the risk of death from novel coronavirus (COVID-19) infection: inference using exported cases. J Clin Med. https://doi.org/10.3390/jcm9020523
Korzeniewska E (2011) Emission of bacteria and fungi in the air from wastewater treatment plants—a review. Front Biosci (Schol Ed). https://doi.org/10.2741/s159
Krammer F (2020) SARS-CoV-2 vaccines in development. Nature 586(7830):516–527. https://doi.org/10.1038/s41586-020-2798-3
Kuo DHW, Simmons FJ, Blair S, Hart E, Rose JB, Xagoraraki I (2010) Assessment of human adenovirus removal in a full-scale membrane bioreactor treating municipal wastewater. Water Res. https://doi.org/10.1016/j.watres.2009.10.039
La Rosa G, Pourshaban M, Iaconelli M, Vennarucci VS, Muscillo M (2010) Molecular detection of hepatitis E virus in sewage samples. Appl Environ Microbiol. https://doi.org/10.1128/AEM.00336-10
La Rosa G, Fratini M, Della Libera S, Iaconelli M, Muscillo M (2013) Viral infections acquired indoors through airborne, droplet or contact transmission. Annali dell’Istituto Superiore di Sanita. https://doi.org/10.4415/ANN-13-02-03
Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR (2020) Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents 55(3):105924. https://doi.org/10.1016/j.ijantimicag.2020.105924
Leung NHL, Chu DKW, Shiu EYC, Chan K-H, McDevitt JJ, Hau BJP, Yen H-L, Li Y, Ip DKM, Peiris JSM, Seto W-H, Leung GM, Milton DK, Cowling BJ (2020) Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. https://doi.org/10.1038/s41591-020-0843-2
Li Y, Qian H, Hang J, Chen X, Hong L, Liang P, Li J, Xiao S, Wei J, Liu L, Kang M (2020a) Evidence for probable aerosol transmission of SARS-CoV-2 in a poorly ventilated restaurant. MedRxiv. https://doi.org/10.1101/2020.04.16.20067728
Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, Zhang HY, Sun W, Wang Y (2020b) COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. https://doi.org/10.1002/jmv.25757
Liu C, Zhou Q, Li Y, Garner LV, Watkins SP, Carter LJ, Smoot J, Gregg AC, Daniels AD, Jervey S, Albaiu D (2020a) Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent Sci 6(3):315–331. https://doi.org/10.1021/acscentsci.0c00272
Liu Y, Ning Z, Chen Y, Guo M, Liu Y, Gali NK, Sun L, Duan Y, Cai J, Westerdahl D, Liu X, Xu K, Ho K, Kan H, Fu Q, Lan K (2020b) Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. https://doi.org/10.1038/s41586-020-2271-3
Lu J, Gu J, Li K, Xu C, Su W, Lai Z, Zhou D, Yu C, Xu B, Yang Z (2020) COVID-19 outbreak associated with air conditioning in restaurant, Guangzhou, China, 2020. Emerg Infect Dis. https://doi.org/10.3201/eid2607.200764
Masclaux FG, Hotz P, Gashi D, Savova-Bianchi D, Oppliger A (2014) Assessment of airborne virus contamination in wastewater treatment plants. Environ Res 133:260–265. https://doi.org/10.1016/j.envres.2014.06.002
Muñoz R, Malhautier L, Fanlo J (2015) Biological technologies for the treatment of atmospheric pollutants. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2015.1055471
Naddeo V (2020) Development of environmental biotechnology and control of emerging biological contaminants: the grand challenge for a sustainable future. Water Environ Res. https://doi.org/10.1002/wer.1439
Naddeo V, Liu H (2020) Editorial perspectives: 2019 novel coronavirus (SARS-CoV-2): what is its fate in urban water cycle and how can the water research community respond? Environ Sci Water Res Technol. https://doi.org/10.1039/d0ew90015j
Nemudryi A, Nemudraia A, Surya K, Wiegand T, Buyukyoruk M, Wilkinson R, Wiedenheft B (2020) Temporal detection and phylogenetic assessment of SARS-CoV-2 in municipal wastewater. MedRxiv. https://doi.org/10.1101/2020.04.15.20066746
Nicastro F, Sironi G, Antonello E, Bianco A, Biasin M, Brucato JR, Ermolli I, Pareschi G, Salvati M, Tozzi P, Trabattoni D, Clerici M (2020) Modulation of COVID-19 epidemiology by UV-B and -A photons from the Sun. arXiv. https://doi.org/10.2139/ssrn.3620694
Park SY, Kim Y-M, Yi S, Lee S, Na B-J, Kim CB, Kim J-I, Kim HS, Kim YB, Park Y, Huh IS, Kim HK, Yoon HJ, Jang H, Kim K, Chang Y, Kim I, Lee H, Gwack J, Jeong EK (2020) Coronavirus disease outbreak in call center, South Korea. Emerg Infect Dis. https://doi.org/10.3201/eid2608.201274
Pastorino B, Touret F, Gilles M, Lamballerie XD, Charrel RN (2020) Evaluation of heating and chemical protocols for inactivating SARS-CoV-2. BioRxiv. https://doi.org/10.1101/2020.04.11.036855
Patel M, Chaubey AK, Pittman CU, Mlsna T, Mohan D (2020) Coronavirus (SARS-CoV-2) in the environment: occurrence, persistence, analysis in aquatic systems and possible management. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2020.142698
Rabenau HF, Cinatl J, Morgenstern B, Bauer G, Preiser W, Doerr HW (2005) Stability and inactivation of SARS coronavirus. Med Microbiol Immunol. https://doi.org/10.1007/s00430-004-0219-0
Randazzo W, Truchado P, Cuevas-Ferrando E, Simón P, Allende A, Sánchez G (2020) SARS-CoV-2 RNA in wastewater anticipated COVID-19 occurrence in a low prevalence area. Water Res. https://doi.org/10.1016/j.watres.2020.115942
Raoult D, Zumla A, Locatelli F, Ippolito G, Kroemer G (2020) Coronavirus infections: epidemiological, clinical and immunological features and hypotheses. Cell Stress. https://doi.org/10.15698/cst2020.04.216
Ratnesar-Shumate S, Williams G, Green B, Krause M, Holland B, Wood S, Bohannon J, Boydston J, Freeburger D, Hooper I, Beck K, Yeager J, Altamura LA, Biryukov J, Yolitz J, Schuit M, Wahl V, Hevey M, Dabisch P (2020) Simulated sunlight rapidly inactivates SARS-CoV-2 on surfaces. J Infect Dis. https://doi.org/10.1093/infdis/jiaa274
Rimoldi SG, Stefani F, Gigantiello A, Polesello S, Comandatore F, Mileto D, Maresca M, Longobardi C, Mancon A, Romeri F, Pagani C, Moja L, Gismondo MR, Salerno F (2020) Presence and vitality of SARS-CoV-2 virus in wastewaters and rivers. MedRxiv. https://doi.org/10.1101/2020.05.01.20086009
Rosa GL, Iaconelli M, Mancini P, Ferraro GB, Veneri C, Bonadonna L, Lucentini L (2020) First detection of Sars-Cov-2 in untreated wastewaters in Italy. MedRxiv. https://doi.org/10.1101/2020.04.25.20079830
Sagripanti JL, Lytle CD (2020) Estimated inactivation of coronaviruses by solar radiation with special reference to COVID-19. Photochem Photobiol. https://doi.org/10.1111/php.13293
Schröder I (2020) COVID-19: a risk assessment perspective. ACS Chem Health Safety. https://doi.org/10.1021/acs.chas.0c00035
Senatore V, Zarra T, Oliva G, Belgiorno V, Naddeo V (2020) Volatile organic compounds (VOCs) control by combining bio-scrubber and ozone pretreatment. Global Nest J 22(2):143–146. https://doi.org/10.30955/gnj.003298
Seto WH (2015) Airborne transmission and precautions: facts and myths. J Hosp Infect. https://doi.org/10.1016/j.jhin.2014.11.005
Setti L, Passarini F, De Gennaro G, Barbieri P, Perrone MG, Borelli M, Palmisani J, Di Gilio A, Torboli V, Fontana F, Clemente L, Pallavicini A, Ruscio M, Piscitelli P, Miani A (2020) SARS-Cov-2RNA found on particulate matter of Bergamo in Northern Italy: first evidence. Environ Res 188(May):109754. https://doi.org/10.1016/j.envres.2020.109754
Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, Wang F, Li D, Yang M, Xing L, Wei J, Xiao H, Yang Y, Qu J, Qing L, Chen L, Xu Z, Peng L, Li Y, Liu L (2020) Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. J Am Med Assoc. https://doi.org/10.1001/jama.2020.4783
Shiraki K, Daikoku T (2020) Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol Ther. https://doi.org/10.1016/j.pharmthera.2020.107512
Van Doremalen N, Bushmake T, Morris DH, Myndi GH, Gamble A, Williamson BN, Tamin A, Lloyd-Smith JO, de Wit E (2020) Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. The N Engl J Med 382:1564–1567
Velavan TP, Meyer CG (2020) The COVID-19 epidemic. Tropical Med Int Health 25(3):278–280. https://doi.org/10.1111/tmi.13383
Wang J, Tang K, Feng K, Lv W (2020a) High temperature and high humidity reduce the transmission of COVID-19. SSRN Electron J. https://doi.org/10.2139/ssrn.3551767
Wang S, Zhang Y, Liu S, Peng H, Mackey V, Sun L (2020) Coronaviruses and the associated potential therapeutics for the viral infections. J Infect Dis Ther 8:2
WHO (2020) Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. Sci Brief. https://doi.org/10.1056/NEJMoa2001316.5
World Health Organization (WHO) (2014) Infection prevention and control of epidemic- and pandemic-prone acute respiratory infections in health care. WHO, Geneva
Wu Y, Guo C, Tang L, Hong Z, Zhou J, Dong X, Yin H, Xiao Q, Tang Y, Qu X, Kuang L, Fang X, Mishra N, Lu J, Shan H, Jiang G, Huang X (2020) Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. https://doi.org/10.1016/S2468-1253(20)30083-2
Wu F, Xiao A, Zhang J, Gu X, Lee WL, Kauffman K, Hanage W, Matus M, Ghaeli N, Endo N, Duvallet C, Moniz K, Erickson T, Chai P, Thompson J, Alm E (2020) SARS-CoV-2 titers in wastewater are higher than expected from clinically confirmed cases. MedRxiv. https://doi.org/10.1101/2020.04.05.20051540
Wurtzer S, Marechal V, Mouchel J-M, Maday Y, Teyssou R, Richard E, Almayrac JL, Moulin L (2020) Evaluation of lockdown impact on SARS-CoV-2 dynamics through viral genome quantification in Paris wastewaters. MedRxiv. https://doi.org/10.1101/2020.04.12.20062679
Zhang B, Liu S, Tan T, Huang W, Dong Y, Chen L, Chen Q, Zhang L, Zhong Q, Zhang X, Zou Y, Zhang S (2020) Treatment with convalescent plasma for critically ill patients with SARS-CoV-2 infection. Chest. https://doi.org/10.1016/j.chest.2020.03.039
Zhao Y, Richardson B, Takle E, Chai L, Schmitt D, Xin H (2019) Airborne transmission may have played a role in the spread of 2015 highly pathogenic avian influenza outbreaks in the United States. Sci Rep 9(1):1–10. https://doi.org/10.1038/s41598-019-47788-z
Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Shi ZL (2020) A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. https://doi.org/10.1038/s41586-020-2012-7
Zhu S, Guo X, Geary K, Zhang D (2020) Emerging therapeutic strategies for COVID-19 patients. Discoveries 8(1):e105. https://doi.org/10.15190/d.2020.2
Funding
Open Access funding provided by Università degli Studi di Salerno. The authors would like express their sincere gratitude for the support provided by (i) the University of Salerno (FARB grants: ORSA11328, 300393FRB18NADDE, and 300393FRB17NADDE), (ii) the Inter-University Centre for Prediction and Prevention of Relevant Hazards (Centro Universitario per la Previsione e Prevenzione Grandi Rischi, C.U.G.RI.), (iii) the National Research Foundation of Korea (grant no. 2019R1H1A2080148), and (iv) the Center for Membranes and Advanced Water Technology (CMAT, Abu Dhabi, UAE).
Author information
Authors and Affiliations
Contributions
The manuscript was written through the contributions of all the authors. All the authors have given their approval to the final version of the manuscript. All authors contributed equally.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Benigno Sanchez-Cabrero, Chief Editor.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Senatore, V., Zarra, T., Buonerba, A. et al. Indoor versus outdoor transmission of SARS-COV-2: environmental factors in virus spread and underestimated sources of risk. Euro-Mediterr J Environ Integr 6, 30 (2021). https://doi.org/10.1007/s41207-021-00243-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41207-021-00243-w