Abstract
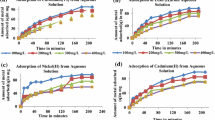
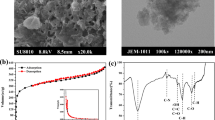
Despite their extensive and decade-long application in wastewater treatment, activated carbon remains one of the most viable adsorbents, with substantive practical application, due to their high pollutant binding capacity. In this study, commercially available activated charcoal was applied in the uptake of aqueous cadmium [Cd(II)] ion. The effect of some process variables on the Cd (II) uptake was investigated via batch mode. Furthermore, the adsorbents’ surface charge (pHPZC), surface morphology (using SEM) and available surface functional groups (using FTIR) were explained. The pH dependence of the present adsorption system was revealed, while the optimum pH was recorded at pH 5.0. Similarly, the Cd (II) uptake (mg/g) decreased with increasing adsorbent dosage due to possible active sites clogging, overcrowding and interference. Furthermore, the isothermal and kinetics analyses of the experimental data, which were aptly validated using the hybrid error model, respectively depicted the Langmuir isotherm and pseudo-second-order kinetic model as the best fit. A Langmuir adsorption capacity of 682.5 mg g−1 was also recorded in the study. Consequently, the present adsorption system was characterized by an equilibrium timeframe of industrial practicability; hence, the adsorbent was successfully applied for the aqueous Cd (II) uptake.






Similar content being viewed by others
Data Availability
Our manuscript has no associated data.
References
Ali, H., E. Khan, and I. Ilahi, Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. Journal of chemistry, 2019. 2019.
Hashem A, Hammad HA, Al-Anwar A (2015) Chemically modified Retama raetam biomass as a new adsorbent for Pb (II) ions from aqueous solution: non-linear regression, kinetics and thermodynamics. Green Processing and Synthesis 4(6):463–478
Usman A, Sallam A, Zhang M, Vithanage M, Ahmad M, Al-Farraj A, Ok YS, Abduljabbar A, Al-Wabel M (2016) Sorption process of date palm biochar for aqueous Cd (II) removal: efficiency and mechanisms. Water Air Soil Pollut 227(12):1–16
Masindi V, Muedi KL (2018) Environmental contamination by heavy metals. Heavy metals 10:115–132
Meseguer VF, Ortuño JF, Aguilar MI, Pinzón-Bedoya ML, Lloréns M, Sáez J, Pérez-Marín AB (2016) Biosorption of cadmium (II) from aqueous solutions by natural and modified non-living leaves of Posidonia oceanica. Environ Sci Pollut Res 23(23):24032–24046
Roe F (1978) International Agency for Research on Cancer (IARC) 1976 annual report. J Clin Pathol 31(2):200
Hashem A, Abdel-Halim E, El-Tahlawy KF, Hebeish A (2005) Enhancement of the adsorption of Co (II) and Ni (II) ions onto peanut hulls through esterification using citric acid. Adsorpt Sci Technol 23(5):367–380
Aniagor CO, Igwegbe CA, Ighalo JO, Oba SN (2021) Adsorption of doxycycline from aqueous media: a review. J Mol Liq. https://doi.org/10.1016/j.molliq.2021.116124:p.116124
Boparai HK, Joseph M, O’Carroll DM (2011) Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J Hazard Mater 186(1):458–465
Tan G, Xiao D (2009) Adsorption of cadmium ion from aqueous solution by ground wheat stems. J Hazard Mater 164(2–3):1359–1363
Pal P, Pal A (2017) Surfactant-modified chitosan beads for cadmium ion adsorption. Int J Biol Macromol 104:1548–1555
Hua, Y., X. Zheng, L. Xue, L. Han, S. He, T. Mishra, Y. Feng, L. Yang, and B. Xing, Microbial aging of hydrochar as a way to increase cadmium ion adsorption capacity: Process and mechanism. Bioresource technology, 2020. 300: p. 122708.
Yusuff AS, Popoola LT, Babatunde EO (2019) Adsorption of cadmium ion from aqueous solutions by copper-based metal organic framework: equilibrium modeling and kinetic studies. Appl Water Sci 9(4):1–11
Hashem, A., S. Badawy, S. Farag, L. Mohamed, A. Fletcher, and G. Taha, Non-linear adsorption characteristics of modified pine wood sawdust optimised for adsorption of Cd (II) from aqueous systems. Journal of Environmental Chemical Engineering, 2020: p. 103966.
Hashem, A., M. Nasr, A. Fletcher, and L.A. Mohamed, Aminated acrylic fabric waste derived sorbent for Cd (II) ion removal from aqueous solutions: mechanism, equilibria and kinetics. Journal of Polymers and the Environment, 2020: p. 1–12.
Abdel-Halim E, Alanazi HH, Al-Deyab SS (2015) Utilization of olive tree branch cellulose in synthesis of hydroxypropyl carboxymethyl cellulose. Carbohyd Polym 127:124–134
Hashem A, Sokker H, Halim EA, Gamal A (2005) γ-induced graft copolymerization onto cellulosic fabric waste for cationic dye removal. Adsorpt Sci Technol 23(6):455–466
Abdel-Halim ES, Al-Hoqbani AA (2015) Utilization of poly (acrylic acid)/cellulose graft copolymer for dye and heavy metal removal. BioResources 10(2):3112–3130
Abdel-Halim E (2012) Physiochemical properties of differently pretreated cellulosic fibers. Carbohyd Polym 88(4):1201–1207
Abdel-Halim ES, Al-Deyab SS, Alfaifi AYA (2014) Cotton fabric finished with β-cyclodextrin: inclusion ability toward antimicrobial agent. Carbohyd Polym 102(1):550–556
ANIAGOR CO, MENKITI MC (2020) Relational description of an adsorption system based on isotherm, adsorption density, adsorption potential, hopping number and surface coverage. Sigma 38(3):1073–1098
Hegyesi N, Vad RT, Pukánszky B (2017) Determination of the specific surface area of layered silicates by methylene blue adsorption: the role of structure, pH and layer charge. Appl Clay Sci 146:50–55
Jia Y, Thomas K (2000) Adsorption of cadmium ions on oxygen surface sites in activated carbon. Langmuir 16(3):1114–1122
Uğurlu M, Gürses A, Açıkyıldız M (2008) Comparison of textile dyeing effluent adsorption on commercial activated carbon and activated carbon prepared from olive stone by ZnCl2 activation. Microporous Mesoporous Mater 111(1–3):228–235
Osasona I, Aiyedatiwa K, Johnson J, Faboya OL (2018) Activated carbon from spent brewery barley husks for cadmium ion adsorption from aqueous solution. Indonesian Journal of Chemistry 18(1):145–152
Nejadshafiee V, Islami MR (2019) Adsorption capacity of heavy metal ions using sultone-modified magnetic activated carbon as a bio-adsorbent. Mater Sci Eng, C 101:42–52
Alkherraz AM, Ali AK, Elsherif KM (2020) Removal of Pb (II), Zn (II), Cu (II) and Cd (II) from aqueous solutions by adsorption onto olive branches activated carbon: equilibrium and thermodynamic studies. Chem Int 6(1):11–20
Manjuladevi M, Anitha R, Manonmani S (2018) Kinetic study on adsorption of Cr (VI), Ni (II), Cd (II) and Pb (II) ions from aqueous solutions using activated carbon prepared from Cucumis melo peel. Appl Water Sci 8(1):1–8
Sharma, G. and M. Naushad, Adsorptive removal of noxious cadmium ions from aqueous medium using activated carbon/zirconium oxide composite: isotherm and kinetic modelling. Journal of Molecular Liquids, 2020. 310: p. 113025.
Xie, X., H. Gao, X. Luo, T. Su, Y. Zhang, and Z. Qin, Polyethyleneimine modified activated carbon for adsorption of Cd (II) in aqueous solution. Journal of Environmental Chemical Engineering, 2019. 7(3): p. 103183.
Zhang, Z., T. Wang, H. Zhang, Y. Liu, and B. Xing, Adsorption of Pb (II) and Cd (II) by magnetic activated carbon and its mechanism. Science of The Total Environment, 2021. 757: p. 143910.
Ioannidou O, Zabaniotou A (2007) Agricultural residues as precursors for activated carbon production—a review. Renew Sustain Energy Rev 11(9):1966–2005
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I. Solids. Journal of the American chemical society 38(11):2221–2295
Igwegbe, C.A., S.N. Oba, C.O. Aniagor, A.G. Adeniyi, and J.O. Ighalo, Adsorption of ciprofloxacin from water: a comprehensive review. Journal of Industrial and Engineering Chemistry, 2020.
Igwegbe CA, Onukwuli OD, Ighalo JO, Okoye PU (2020) Adsorption of cationic dyes on Dacryodes edulis seeds activated carbon modified using phosphoric acid and sodium chloride. Environmental Processes 7(4):1151–1171
Wohleber DA, Manes M (1971) Application of the Polanyi adsorption potential theory to adsorption from solution on activated carbon. III. Adsorption of miscible organic liquids from water solution. The Journal of Physical Chemistry 75(24):3720–3723
Freundlich H (1907) Über die adsorption in lösungen. Z Phys Chem 57(1):385–470
Temkin M (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta physiochim URSS 12:327–356
Aniagor C, Menkiti M (2018) Kinetics and mechanistic description of adsorptive uptake of crystal violet dye by lignified elephant grass complexed isolate. J Environ Chem Eng 6(2):2105–2118
Lagergren SK (1898) About the theory of so-called adsorption of soluble substances. Sven Vetenskapsakad Handingarl 24:1–39
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34(5):451–465
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89(2):31–60
Kumar KV, Sivanesan S (2006) Pseudo second order kinetics and pseudo isotherms for malachite green onto activated carbon: comparison of linear and non-linear regression methods. J Hazard Mater 136(3):721–726
Menkiti M, Abonyi M, Aniagor C (2018) Process equilibrium, kinetics, and mechanisms of ionic-liquid induced dephenolation of petroleum effluent. Water Conservation Science and Engineering 3(3):205–220
El-Khaiary MI, Malash GF (2011) Common data analysis errors in batch adsorption studies. Hydrometallurgy 105(3–4):314–320
Menkiti M, Aniagor C, Agu C, Ugonabo V (2018) Effective adsorption of crystal violet dye from an aqueous solution using lignin-rich isolate from elephant grass. Water Conservation Science and Engineering 3(1):33–46
Nandiyanto ABD, Oktiani R, Ragadhita R (2019) How to read and interpret FTIR spectroscope of organic material. Indonesian Journal of Science and Technology 4(1):97–118
Conley RT, Bieron JF (1963) Examination of the oxidative degradation of polyacrylonitrile using infrared spectroscopy. J Appl Polym Sci 7(5):1757–1773
Ngah WW, Teong L, Toh R, Hanafiah M (2012) Utilization of chitosan–zeolite composite in the removal of Cu (II) from aqueous solution: adsorption, desorption and fixed bed column studies. Chem Eng J 209:46–53
Nwosu FO, Ajala OJ, Owoyemi RM, Raheem BG (2018) Preparation and characterization of adsorbents derived from bentonite and kaolin clays. Appl Water Sci 8(7):1–10
Hashem A, Aniagor C, Taha G, Fikry M (2021) Utilization of low-cost sugarcane waste for the adsorption of aqueous Pb(II): kinetics and isotherm studies. Current Research in Green and Sustainable Chemistry. https://doi.org/10.1016/j.crgsc.2021.100056
Hashem A, Abou-Okeil A, Fikry M, Aly A, Aniagor CO (2021) Isotherm and kinetics parametric studies for aqueous Hg(II) uptake onto N-[2-(methylamino)ethyl]ethane-1,2-diaminated acrylic fibre. Arab J Sci Eng. https://doi.org/10.1007/s13369-021-05416-x
Hamdaoui O (2006) Batch study of liquid-phase adsorption of methylene blue using cedar sawdust and crushed brick. J Hazard Mater 135(1–3):264–273
Hashem, A., C.O. ANIAGOR, M. Nasr, and A. Abou-Okeil, Efficacy of treated sodium alginate and activated carbon fibre for Pb(II) adsorption. International journal of biological macromolecules, 2021. https://doi.org/10.1016/j.ijbiomac.2021.02.067: p. 1–16.
Chen H, Zhao Y, Wang A (2007) Removal of Cu (II) from aqueous solution by adsorption onto acid-activated palygorskite. J Hazard Mater 149(2):346–354
Aniagor C, Abdel-Halim E, Hashem A (2021) Evaluation of the aqueous Fe (II) ion sorption capacity of functionalized microcrystalline cellulose. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2021.105703:p.105703
Alhan S, Nehra M, Dilbaghi N, Singhal NK, Kim K-H, Kumar S (2019) Potential use of ZnO@ activated carbon nanocomposites for the adsorptive removal of Cd2+ ions in aqueous solutions. Environ Res 173:411–418
Kannan N, Sundaram MM (2001) Kinetics and mechanism of removal of methylene blue by adsorption on various carbons—a comparative study. Dyes Pigm 51(1):25–40
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aniagor, C.O., Elshkankery, M., Fletcher, A.J. et al. Equilibrium and Kinetic Modelling of Aqueous Cadmium Ion and Activated Carbon Adsorption System. Water Conserv Sci Eng 6, 95–104 (2021). https://doi.org/10.1007/s41101-021-00107-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41101-021-00107-y