Abstract
Introduction
Inhaled drugs are essential for treatment of asthma and chronic obstructive pulmonary disease, but drug delivery via the respiratory tract is complex. Correct inhaler use is challenging as inhalers differ and inhaler technique errors are common. This in vitro study examined impact of inhalation flow, volume, and shaking of commonly used budesonide/formoterol (BUD/FORM) dry powder inhalers (DPIs).
Methods
Two strengths of each included BUD/FORM DPI, Turbuhaler, Easyhaler and Spiromax, were examined. Fine particle dose (FPD) was measured using the Next Generation Impactor, at fixed pressure drops of 2, 4 and 6 kPa. Delivered dose (DD) for inhalation volume and shaking tests was performed at an airflow rate equivalent to a 4-kPa pressure drop, collected on Respirgard II, 303EU filters.
Results
FPD flow rate dependence was similar for all inhalers and strengths. BUD/FORM Turbuhaler and Easyhaler were unaffected by inhalation volume, whereas Spiromax showed significant inhalation volume dependence with about 50% decrease for both inhaler strengths. For a BUD/FORM Turbuhaler 160/4.5-µg/dose, there were no differences in the DD between shaking and no shaking, whereas the higher strength showed a 10% decrease after shaking. BUD/FORM Easyhaler DD decreased with about 50% for both strengths when the device was not shaken, while Spiromax decreased with 20% for the lower strength and 80% for the higher strength when shaken.
Conclusion
This study revealed significant differences in inhaler performance after testing at different inhalation flow and volume and following exposure to potential pre-inhalation handling errors, which may lead to decreased drug delivery to the lungs.
Funding
AstraZeneca
Similar content being viewed by others
Introduction
Inhaled drugs are essential for the treatment of asthma and chronic obstructive pulmonary disease (COPD) [1, 2], but drug delivery via the respiratory tract is more complex than oral therapy. Correct use of the inhaler is necessary to enable the medication to be effectively delivered to the lungs with maximized clinical efficacy and minimized systemic side effects [3]. Still, a significant proportion of patients makes inhaler technique errors [3–5]. Generic inhalation instructions apply to all inhalers; to exhale before the inhalation and to inhale as deeply as possible; slow through a pressurized metered dose inhaler (pMDI), and fast through a dry powder inhaler (DPI) [6]. Also, most pMDIs should be shaken before use, while the majority of DPIs should not [7]. This in vitro study evaluates the impact of potential patient mishandling errors on the performance of different inhalers.
In the Nordic countries, the majority of treatments with fixed or free combinations of inhaled corticosteroids and long-acting beta2-agonists are delivered by a DPI [8]. However, there are differences between DPIs, e.g. in the formulation of the drug. The fine particle dose (FPD) dependency of the inhalation air flow rate is a fundamental characteristic of DPIs. Depending on the design of the device, the properties of the dry powder formulation and the interaction between these, DPIs may have different inhalation flow rate dependency profiles. Further, the patient’s inhalation volume is an important patient-related factor when selecting an inhaler. The inhalation volume needed for complete dose emission and, consequently, delivered dose (DD) from the inhaler, may vary between devices. Hence, a low inhalation volume could compromise the DD [9].
In addition, each device has its own specific instructions on how to prepare the dose for reliable drug delivery, e.g. removing the protective cap, pushing a lever, or specific preparations such as shake or not shake the inhaler before use [7]. Differences in shelf life, time in-use [10] and storage conditions such as protection against humidity [8] should also be considered. Hence, the device should be selected based on patient’s characteristics, the likelihood of success with the inhalation manoeuvres, inhalation technique and patient preference [11].
The aim of this in vitro study was to examine the impact of inhalation flow, inhalation volume and handling errors on the performance of three commonly used budesonide/formoterol (BUD/FORM) DPIs.
Methods
Materials
Three medium-resistance (60–70 Pa0.5 s L−1) reservoir multi-dose DPIs containing fixed combinations of BUD/FORM inhalers were tested: BUD/FORM Turbuhaler 160/4.5 µg/dose, 120 doses and BUD/FORM Turbuhaler 320/9 µg/dose, 60 doses (AstraZeneca, Södertälje, Sweden); BUD/FORM Spiromax 160/4.5 µg/dose, 120 doses and BUD/FORM Spiromax 320/9 µg/dose, 60 doses (TEVA Pharmaceutical, Petach Tikva, Israel); and BUD/FORM Easyhaler 160/4.5 µg/dose, 120 doses and BUD/FORM Easyhaler 320/9 µg/dose, 60 doses (Orion Pharma, Espoo, Finland). All samples had at least 9 months of shelf life remaining when tested. The inhalers are hereafter referred to by their respective trade name.
Cascade Impactor Analysis (Flow Rate Dependency)
The FPD was measured using the Next Generation Impactor (NGI) [12, 13] operating at fixed pressure drops of 2, 4 and 6 kPa. The flow rates obtained were 45, 64 and 78 L/min, respectively, for Turbuhaler and Spiromax, and 40, 56 and 69 L/min, respectively, for Easyhaler. The NGI was equipped with a pre-separator. The volume of air drawn through the inhalers and the NGI was fixed to 4 L. The FPD is defined as the amount of active pharmaceutical ingredient (API) contained in particles <5 µm in size and the amount was calculated using interpolation between relevant stages which depend on the flow rate (stage cut-offs vary with varying flow rate). For each product and flow rate, three inhalers were tested. For each inhaler, doses 1–6 were analyzed.
Inhalation Volume Test
Three samples from each of the inhalers were tested at 0.25-, 0.5-, 1.0-, 2.0- and 4.0-L inhalation volume. For each inhaler, doses 1–3 were wasted using a 4-L inhalation volume. Doses 4, 6, 8, 10 and 12 were tested for each of the different inhalation volumes. Doses 5, 7, 9 and 11 were wasted using 4-L inhalation volume in order to eliminate any memory effect from the previous dose.
Shaking Test
Doses 1–5, 58–62 and 116–120 were tested for the 160/4.5-µg products and doses 1–5, 28–32 and 56–60 were tested for the 320/9-µg products. The inhalation volume was 4 L [14]. Shaking of each tested device was performed manually by an experienced analyst, with five shakes (down–up–down–up–down) of 3–4 s in duration, with a shake length of approximately 0.15 m. This is consistent with the shaking required for effective use of a pMDI and can be considered as representative of a likely patient error in a real-world setting. All inhalers were shaken in the same way. For each product and strength, five inhalers were tested with shaking and five inhalers without shaking. Turbuhaler and Easyhaler were shaken before loading the dose. This is in accordance with the Easyhaler patient instruction; there is no mentioning of shaking in the Turbuhaler instruction. Spiromax was shaken after the dose loading (opening of the cap) which is at odds with the patient instruction to not shake.
Delivered Dose Testing
Both the inhalation volume test and the shaking test were carried out at an airflow rate equivalent to a 4-kPa pressure drop over the inhalers, i.e. 64 L/min for both Turbuhaler and Spiromax and 56 L/min for BUD/FORM Easyhaler. The doses were collected on Respirgard II, 303EU filters. The filters were extracted with a suitable solvent (50% methanol in water) containing an internal standard (butyl 4-hydroxybenzoate) and the sample solutions were quantified by isocratic reversed-phase liquid chromatography. Both budesonide and formoterol were measured.
All tests were performed by an independent laboratory.
Statistical Analyses
For each product and for each API, the effect of inhalation volume was investigated. The results obtained at 0.25, 0.5, 1.0 and 2.0 L were compared to the results obtained at 4.0 L using a paired t test. Bonferroni’s correction for multiple tests was applied [15] to declare statistical significance with an overall alpha level of 0.05 (0.05/4 = 0.0125 for each comparison).
Compliance with Ethics Guidelines
As an in vitro study, this article does not contain any new studies with human or animal subjects performed by any of the authors.
Results
Flow Rate Dependency of FPD
The FPD ratio of low flow vs. medium flow was consistently about 0.8 for all inhalers and strengths, and for both APIs. Similarly, the ratio of high flow vs. medium flow was about 1.1. While the FPD results for budesonide were reasonably consistent between the inhalers and within strengths (Fig. 1), the FPD results for formoterol were consistently higher for Turbuhaler compared to the analogues (Fig. 2).
Effects of Inhalation Volume
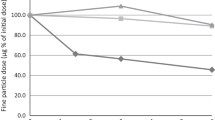
For Turbuhaler, the DD was unaffected by the inhalation volume down to 1 L. For the lower volumes, the DD decreased by about 10%, which was statistically significant for 0.25 L (both strengths) and for 0.5 L (only the higher strength; Fig. 3). For Easyhaler, the DD was unaffected for all volumes down to 0.5 L and showed an approximate 10% decrease at 0.25 L, although not statistically significant except for the formoterol component in the lower strength (Fig. 3). The delivered dose for Spiromax showed a significant inhalation volume dependence. At the lowest volume, 0.25 L, the budesonide and formoterol DD values for the 160/4.5-µg strength was about 50% of the value obtained at 4-L volume. There were statistically significant differences for all tested volumes compared to the 4-L volume. A similar trend was observed for the 320/9-µg strength although the 2-L values were not statistically different from the 4-L values. At the lowest volume, 0.25 L, the budesonide and formoterol DD values were about 40% of the value obtained at the 4-L volume (Fig. 3).
Effects of Shaking and No Shaking
For a Turbuhaler 160/4.5-µg/dose, there were no differences in the DD between shaking and no shaking for any of the APIs (Table 1). For the higher strength, 320/9-µg/dose, a small decrease in DD, approximately 10% for both APIs, was observed after shaking compared to no shaking (Fig. 4a). For Easyhaler, the DD for both APIs and for both strengths decreased by about 50% when the device was not shaken (Fig. 4a, b). The results for Spiromax indicated that the two strengths were impacted by shaking to a different extent. The lower strength showed an approximate 20% decrease in DD for both APIs when shaken compared to not shaken (Table 1), while for the higher-strength dose, the decrease was about 80% for both APIs when the device was shaken (Fig. 4c).
Discussion
In this in vitro study, we found that the three inhalers tested were equally flow-dependent regarding the FPD of budesonide and formoterol. The Turbuhaler and Easyhaler inhalers showed non-dependency of inhalation volume for DD, whereas Spiromax had a clinically relevant inhalation volume dependency for DD when inhaling with low inhalation volume. Further, we found that there were major differences in inhaler performance when inhaler-specific pre-inhalation instructions were not followed. The present study reports that Spiromax DD decreased by 20% and 80% for the low and the high strengths, respectively, when shaken before use, whereas Easyhaler decreased by 50% for both strengths if it was not shaken prior to use. The DD from the Turbuhaler was similar, regardless if shaken or not.
When comparing inhalation devices, the most common parameter that previously has been evaluated is the flow dependency of FPD. The flow rate dependency for Turbuhaler devices has been extensively studied [16, 17]. For analogues to be approved in the European Union (EU), it is a prerequisite that the particle size distribution is similar to the originator product at three different flow rates: low, medium and high [18]. The EU approvals of the BUD/FORM Turbuhaler analogue products, Easyhaler and Spiromax, were based on pharmacokinetic data and by showing that the flow rate dependency of FPD was similar to the originator product [19, 20]. A previous study, comparing Turbuhaler and Easyhaler, had shown similar flow rate dependency of the devices [21]. In that study, the flow rate ranges investigated were shifted upwards, 45–75 L/min for Easyhaler and 55–93 L/min for Turbuhaler, compared to the present study. The pharmacopeia standard test condition at 4 kPa was used as the medium flow rate and the low and high flow rates, equivalent to 2 and 6 kPa, respectively, were selected in accordance with De Boer et al. [16]. The results in our study showed a similar FPD flow rate dependency between the three inhalers tested.
Data regarding the impact of inhalation volume is scarce. The present study reports on an apparent inhalation volume dependency for DD for Spiromax, whereas Turbuhaler and Easyhaler showed non-dependency of inhalation volume, a difference that may have impact on clinical outcomes. Patients with COPD have significantly lower inhalation volumes, approximately 2 L, compared to healthy subjects [22–26]. A study by Seheult reported that the mean inspiratory vital capacity (IVC) was 2.13 L (±0.79) [23], whereas Azous et al. reported an IVC of 1.82 L (±0.88) in patients with moderate COPD (FEV1 52% predicted normal), and 1.90 L (±0.90) after enhanced training [26]. Important to note is also the decline of the inhalation volume as the disease progresses. In severe and very severe COPD, the inhalation volume has been reported to decrease to 1.7 L (CI 1.4–2.1) and 1.3 L (CI 1.1–1.5), respectively [24]. Low inhalation volumes in patients using volume-dependent inhalers may result in the inhalation volume being too low to allow drug emission. Volume dependence may also have an impact in children and adolescents with asthma, since this population have considerably lower inhalation volumes, 1.50 L (0.6) and 2.03 L (0.81), respectively [26].
In addition, studies have shown that one third of the patients fail to exhale and inhale deep before inhalation, leading to additional decrease of the actual inhalation volume [5]. In this context it should be noted that the delivered dose test volume described in the European Pharmacopeia, developed for quality control and registration purposes, is 4 L. Thus, a volume dependence of an inhaler would not be detected during routine quality testing [12].
To our knowledge, the impact of shaking or not shaking the device before use has not been previously reported. The present study showed that Turbuhaler was virtually unaffected by shaking while both Easyhaler and Spiromax were severely affected when the products were either shaken (Spiromax) or not shaken (Easyhaler). There is no mentioning of shaking before use in the Turbuhaler patient information leaflet, only that it should be held upright when loading a dose. The Easyhaler patient information leaflet, however, states that the device should be shaken vigorously up and down 3–5 times before use. This is contrary to Spiromax, which should not be shaken according to the patient information leaflet. It has been shown in several studies that simultaneous use of different types of inhaler device, particularly a mixture of pMDI and DPI inhalers, is predictive of increased errors in inhalation [27, 28]. It has also been reported that up to 37% of the patients fail to shake their pMDI before inhalation [3, 29, 30]. Hence, if Spiromax is concomitantly used with a pMDI, the risk of confusion is obvious.
This study comes with several limitations, the major being that it was conducted in vitro. The impact of real-life conditions in everyday patient use were not tested, thereby excluding other potential handling errors or patient-related factors, which may have affected the results. Further, the clinical implications associated with the findings in this in vitro study have not been investigated; however, the magnitude of decrease in DD and FPD observed for some of the inhalers tested may have an impact on clinical outcomes.
High rates of incorrect inhaler technique have been reported [31] and incorrect inhaler technique is considered as a common cause of uncontrolled asthma [3, 32]. Severe exacerbations and asthma-related hospitalizations have been reported to be significantly more common among patients making ≥1 critical inhaler error, compared to those making no errors [5]. Even after meticulous instructions to “mastery” in inhaler technique, the erosion of patient compliance to inhalation instructions is fast. Already one month after initial instructions, only 60% of the patients used the inhalers correctly [33]. The Global Strategy for Asthma Management and Prevention (updated in 2016), states that up to 80% of asthma patients do not use their inhaler correctly [1]. In addition, it has been shown that exposure to a new inhaler device without training is associated with decreased asthma control [34, 35].
The decrease in DD and FPD observed for some of the inhalers in the present study may have clinical implications in patients with low inhalation capacity or patients non-adherent to inhaler instructions.
The role of the health care providers in inhaler use is, therefore, critical, both in achieving initial correct inhaler technique and also in maintaining correct inhaler use over time.
Conclusions
While there are similarities between the tested DPIs regarding the generic inhalation maneuver needed to deliver budesonide and formoterol, there are differences in the device-specific pre-inhalation instructions. This study revealed significant differences in inhaler performance after testing at different inhalation flows and volumes, and exposure to potential pre-inhalation handling errors, which may lead to decreased drug delivery to the lungs. The choice of inhaler should be individualized, as not all inhalers are suitable for all patients. Instructions for optimal inhalation technique and specific device handling need to be in focus, both for health care professionals and for patients.
References
GINA report, Global Strategy for Asthma Management and Prevention. 2016. www.ginasthma.org.
Global strategy for the diagnosis, management and prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2015. www.goldcopd.org.
Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105(6):930–8.
Price D, Bosnic-Anticevich S, Briggs A. Inhaler competence in asthma: common errors, barriers to use and recommended solutions. Respir Med. 2013;107(1):37–46.
Westerik JA, Carter V, Chrystyn H. Characteristics of patients making serious inhaler errors with a dry powder inhaler and association with asthma-related events in a primary care setting. J Asthma. 2016;53(3):321–9.
Chrystyn H, Price D. What you need to know about inhalers and how to use them. Prescriber. 2009;20:47–52.
Levy ML, Dekhuijzen PN, Barnes PJ. Inhaler technique: facts and fantasies. A view from the Aerosol Drug Management Improvement Team (ADMIT). NPJ Prim Care. Respir Med. 2016;21(26):16017. doi:10.1038/npjpcrm.2016.17.
Janson C, Lööf T, Telg G, et al. Difference in resistance to humidity between commonly used dry powder inhalers: an in vitro study. NPJ Prim Care Respir Med. 2016;17(26):16053.
Laube BL, Janssens HM, de Jongh FHC, et al. ERS/ISAM Task force report. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J. 2011;37:1308–31.
Norderud Lærum B, Telg G, Stratelis G. Need of education for dry powder inhaler storage and retention: a patient-reported survey. Multidiscip Respir Med. 2016;8(11):21.
Horne R, Price D, Cleland J. Can asthma control be improved by understanding the patient’s perspective? BMC Pulmon Med. 2007;7:8.
European Directorate for Quality in Medicines and Healthcare. Preparations for inhalation: aerodynamic assessment of fine particles. Strasbourg: European Pharmacopeia 8th Edition; 2014. p. 316–9.
United States Pharmacopeia: USP38, Chapter 601: Physical tests and determinations; Inhalation and Nasal Drug Products: pp. 410–413.
European Directorate for Quality in Medicines and Healthcare. European Pharmacopeia 8th Edition: Strasbourg 2014; p. 804.
Bland JM, Altman DG. Multiple significance tests: the Bonferroni method. BMJ. 1995;. doi:10.1136/bmj.310.6973.170.
de Boer AH, Gjaltema D, Hagedoorn P. Can extrafine dry powder aerosols improve lung deposition. Eur J Pharm Biopharm. 2015;96:143–51.
Buttini F, Brambilla G, Copelli D. Effect of flow rate on in vitro aerodynamic performance of NEXThaler in Comparison with diskus and turbohaler dry powder inhalers. J Aerosol Med Pulm Drug Del. 2016;29(2):167–78.
EMEA guideline on the investigation of bioequivalence. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500070039.pdf. Accessed 12 March 2015.
Bufomix Easyhaler Public Assessment Report, SE/H/1213/02-03/DC, IV-5, p 8(10).
Duoresp Spiromax, CHMP Assessment Report, EMA/CHMP/175692/2014, pp. 64–67.
Malmberg PL, Everard ML, Haikarainen J, Lahelma S. Evaluation of in vitro and in vivo flow rate dependency of budesonide/formoterol easyhaler. J Aerosol Med Pulm Drug Del. 2014;27:1–12.
Lexmond A, Kruizinga TJ, Hagedoorn P, et al. Effect of inhaler design variables on paediatric use of dry powder inhalers. PLOS One. 2014;9(6):e99304.
Seheult JN, Costello S, Tee KC. Investigating the relationship between peak inspiratory flow rate and volume of inhalation from a Diskus™ Inhaler and baseline spirometric parameters: a cross-sectional study. SpringerPlus. 2014;3:496.
Prime D, de Backe WR, Hamilton M, et al. Effect of disease severity in asthma and chronic obstructive pulmonary disease on inhaler-specific inhalation profiles through the ELLIPTA dry powder inhaler. J Aerosol Med Pulm Drug Del. 2015;28(6):1–12.
Azouz W, Campbell J, Stephenson J, et al. Improved metered dose inhaler technique when a coordination cap is used. J Aerosol Med Pulm Drug Del. 2014;27(3):193–9.
Azouz W, Chetcuti P, Hosker H. Inhalation characteristics of asthma patients, COPD patients and healthy volunteers with the Spiromax® and Turbuhaler® devices: a randomized, cross-over study. BMC Pulm Med. 2015;15:47.
van der Palen J, Klein JJ, van Herwaarden CL. Multiple inhalers confuse asthma patients. Eur Respir J. 1999;14:1034–7.
Rootmensen GN, van Keimpema AR, Jansen HM. Predictors of incorrect inhalation technique in patients with asthma or COPD: a study using a validated videotaped scoring method. J. Aerosol Med. Pulm. Drug Del. 2010;23:323–8.
Molimard M, Raherison C, Lignot S. Assessment of handling of inhaler devices in real life: an observational study in 3811 patients in primary care. J Aerosol Med. 2003;16:249–54.
Molimard M. How to achieve good compliance and adherence with inhalation therapy. Curr Med Res Op. 2005;21(4):S33–7.
Fink JB, Rubin BK. Problems with inhaler use: a call for improved clinician and patient education. Respir Care. 2005;50:1360–74.
Haughney J, Price D, Kaplan A. Achieving asthma control in practice: understanding the reasons for poor control. Respir Med. 2008;102:1681–93.
Ovchinikova LB, Smith LBA, Bosnic-Anticevich S. Inhaler technique maintenance: gaining an understanding from the patient’s perspective. J Asthma. 2011;48(6):616–24.
Ekberg-Jansson A, Svenningsson I, Ragdell P. Budesonide inhaler device switch patterns in an asthma population in Swedish clinical practice (ASSURE). Int J Clin Prac. 2015;69(10):1171–8.
Thomas M, Price D, Chrystyn H, et al. Inhaled corticosteroids for asthma: impact of practice level device switching on asthma control. BMC Pulm Med. 2009;9:1.
Acknowledgements
The in vitro testing was performed by Emmace Consulting AB, Lund, Sweden. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity for the work as a whole, and have given final approval for the version to be published. The study was sponsored by AstraZeneca, who also funded the journal’s article processing charges.
Disclosures
Christer Janson has received honoraria for educational activities from AstraZeneca, Boehringer Inelheim, Chiesi, Novartis and TEVA and and for advisory board meetings arranged by AstraZeneca, Boehringer Ingelheim, GSK and TEVA. Thomas Lööf is a full time employee of AstraZeneca, the study sponsor. Georgios Stratelis is a full time employee of AstraZeneca, the study sponsor. Gunilla Telg is a full time employee of AstraZeneca, the study sponsor.
Author Contributions
Thomas Lööf, Georgios Stratelis and Gunilla Telg participated equally in the study design. Thomas Lööf was responsible for statistical analyses. Gunilla Telg drafted the manuscript. All authors analyzed and interpreted the data, revised the manuscript, had access to complete study data and had authority over manuscript preparation, approval of the final version and the decision to submit for publication. Christer Janson is the guarantor.
Compliance with Ethics Guidelines
As an in vitro study, this article does not contain any new studies with human or animal subjects performed by any of the authors.
Data Availability
The datasets generated during the current study are not publicly available, being intellectual property of the study sponsor.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
The original version of this article was revised: The heading ‘RESULTS’ was missing following the ‘Compliance with Ethics Guidelines’ section and has been added.
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/7C08F06067AEA3D4.
An erratum to this article is available at http://dx.doi.org/10.1007/s41030-017-0045-3.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Janson, C., Lööf, T., Telg, G. et al. Impact of Inhalation Flow, Inhalation Volume and Critical Handling Errors on Delivered Budesonide/Formoterol Dose in Different Inhalers: An In Vitro Study. Pulm Ther 3, 243–253 (2017). https://doi.org/10.1007/s41030-017-0042-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41030-017-0042-6