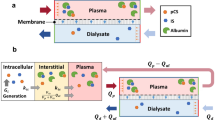
Abstract
Hemodialysis constitutes the lifeline of patients with end stage renal disease, yet the parameters that affect hemodialyzer performance remain incompletely understood. We developed a computational model of mass transfer and solute transport in a hollow-fiber dialyzer to gain greater insight into the determinant factors. The model predicts fluid velocity, pressure, and solute concentration profiles for given geometric characteristics, membrane transport properties, and inlet conditions. We examined the impact of transport and structural parameters on uremic solute clearance by varying parameter values within the constraints of standard clinical practice. The model was validated by comparison with published experimental data. Our results suggest solute clearance can be significantly altered by changes in blood and dialysate flow rates, fiber radius and length, and net ultrafiltration rate. Our model further suggests that the main determinant of the clearance of unreactive solutes is their diffusive permeability. The clearance of protein-bound toxins is also strongly determined by blood hematocrit and plasma protein concentrations. Results from this model may serve to optimize hemodialyzer operating conditions in clinical practice to achieve better clearance of pathogenic uremic solutes.
Lay Summary
There are nearly 500,000 patients in the USA on kidney dialysis, and a large percentage of these patients use hollow-fiber dialyzers; yet, there is much room for improvement of their performance. To address this issue, we developed a computational model to understand the transport properties of hollow-fiber dialyzers and their effects on clearance of toxins. This study is inspired by the early work of Robert S. Langer in the area of immobilized heparinase in extracorporeal devices, and we continue to look to him as an inspiration for translational research to—in his words—“make a positive impact to improve the quality of life”.





Similar content being viewed by others
References
Saran R, et al. US Renal Data System 2017 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2018;71(3S1):A7.
Vanholder R, Schepers E, Pletinck A, Nagler EV, Glorieux G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: a systematic review. J Am Soc Nephrol. 2014;25(9):1897–907.
Liabeuf S, Drueke TB, Massy ZA. Protein-bound uremic toxins: new insight from clinical studies. Toxins (Basel). 2011;3(7):911–9.
Niwa T. Targeting protein-bound uremic toxins in chronic kidney disease. Expert Opin Ther Targets. 2013;17(11):1287–301.
Vanholder R, Glorieux G. Introduction: uremic toxicity - state of the art 2014. Semin Nephrol. 2014;34(2):85–6.
Shashar M, Francis J, Chitalia V. Thrombosis in the uremic milieu--emerging role of "thrombolome". Semin Dial. 2015;28(2):198–205.
Kolachalama VB, Shashar M, Alousi F, Shivanna S, Rijal K, Belghasem ME, et al. Uremic solute-aryl hydrocarbon receptor-tissue factor axis associates with thrombosis after vascular injury in humans. J Am Soc Nephrol. 2018;29(3):1063–72.
Shashar M, et al. Targeting STUB1-tissue factor axis normalizes hyperthrombotic uremic phenotype without increasing bleeding risk. Sci Transl Med. 2017:9(417).
Meyer TW, Walther JL, Pagtalunan ME, Martinez AW, Torkamani A, Fong PD, et al. The clearance of protein-bound solutes by hemofiltration and hemodiafiltration. Kidney Int. 2005;68(2):867–77.
Meert N, et al. Effective removal of protein-bound uraemic solutes by different convective strategies: a prospective trial. Nephrol Dial Transplant. 2009;24(2):562–70.
Jaffrin MY, Gupta BB, Malbrancq JM. A one-dimensional model of simultaneous hemodialysis and ultrafiltration with highly permeable membranes. J Biomech Eng. 1981;103(4):261–6.
Werynski A, Waniewski J. Theoretical description of mass transport in medical membrane devices. Artif Organs. 1995;19(5):420–7.
Pallone TL, Hyver S, Petersen J. The simulation of continuous arteriovenous hemodialysis with a mathematical model. Kidney International. 1989;35(1):125–33.
Donato D, et al. Optimization of dialyzer design to maximize solute removal with a two-dimensional transport model. J Membrane Sci. 2017;541:519–28.
Kedem O, Katchalsky A. Thermodynamic analysis of the permeability of biological membranes to non-electrolytes. Biochimica et Biophysica Acta. 1958;27:229–46.
Liao Z, Poh CK, Huang Z, Hardy PA, Clark WR, Gao D. A numerical and experimental study of mass transfer in the artificial kidney. J Biomech Eng. 2003;125(4):472–80.
Lee JC, Lee K, Kim HC. Mathematical analysis for internal filtration of convection-enhanced high-flux hemodialyzer. Comput Methods Programs Biomed. 2012;108(1):68–79.
Yamamoto K, Matsuda M, Hirano A, Takizawa N, Iwashima S, Yakushiji T, et al. Computational evaluation of dialysis fluid flow in dialyzers with variously designed jackets. Artif Organs. 2009;33(6):481–6.
Aniort J, Chupin L, Cindea N. Mathematical model of calcium exchange during haemodialysis using a citrate containing dialysate. Math Med Biol. 2018;35(suppl_1):87–120.
Viaene L, Annaert P, de Loor H, Poesen R, Evenepoel P, Meijers B. Albumin is the main plasma binding protein for indoxyl sulfate and p-cresyl sulfate. Biopharm Drug Dispos. 2013;34(3):165–75.
Yi D, et al. Determination of the binding properties of p-cresyl glucuronide to human serum albumin. Biochimie. 2018;150:1–7.
Patlak CS, Goldstein DA, Hoffman JF. The flow of solute and solvent across a two-membrane system. J Theor Biol. 1963;5(3):426–42.
Annan K. Mathematical modeling for hollow fiber dialyzer: blood and HCO 3 - -dialysate flow characteristics. IJPAM. 2012;79:425–52.
Landis, E.M. and J.F. Pappenheimer, Exchange of substances through the capillary walls, in Handbook of physiology. 1963, American Physiological Society: Washington, DC. p. pp. 961-1034.
Hall, J.E., Guyton and Hall textbook of medical physiology. 13 ed. 2016, Philadelphia, PA: Elsevier.
Pallone TL, Petersen J. A mathematical model of continuous arteriovenous hemofiltration predicts performance. ASAIO Trans. 1987;33(3):304–8.
Lightfoot EN. Transport phenomena and living systems—biomedical aspects of momentum and mass transport. New York City, NY: Wiley; 1974.
Preston, B.N., et al., Diffusion of dextran at intermediate concentrations. Journal of the Chemical Society, Faraday Transactions 1: Physical Chemistry in Condensed Phases, 1982. 78(4): p. 1209-1221.
Collins MC, Ramirez WF. Transport through polymeric membranes. J Phys Chem. 1979;83(17):2294–301.
Liao Z, et al. Measurement of hollow fiber membrane transport properties in hemodialyzers. J Membrane Sci. 2005;256(1):176–83.
Luo FJ, Patel KP, Marquez IO, Plummer NS, Hostetter TH, Meyer TW. Effect of increasing dialyzer mass transfer area coefficient and dialysate flow on clearance of protein-bound solutes: a pilot crossover trial. Am J Kidney Dis. 2009;53(6):1042–9.
Sirich TL, Funk BA, Plummer NS, Hostetter TH, Meyer TW. Prominent accumulation in hemodialysis patients of solutes normally cleared by tubular secretion. J Am Soc Nephrol. 2014;25(3):615–22.
Eloot S, Schneditz D, Cornelis T, van Biesen W, Glorieux G, Dhondt A, et al. Protein-bound uremic toxin profiling as a tool to optimize hemodialysis. PLOS ONE. 2016;11(1):e0147159.
Poesen R, et al. The influence of renal transplantation on retained microbial-human co-metabolites. Nephrol Dial Transplant. 2016;31(10):1721–9.
Etinger A, Kumar SR, Ackley W, Soiefer L, Chun J, Singh P, et al. The effect of isohydric hemodialysis on the binding and removal of uremic retention solutes. PLOS ONE. 2018;13(2):e0192770.
Poesen R, Evenepoel P, de Loor H, Kuypers D, Augustijns P, Meijers B. Metabolism, protein binding, and renal clearance of microbiota-derived p-cresol in patients with CKD. Clin J Am Soc Nephrol. 2016;11(7):1136–44.
Schneditz D. TMP revisited: the importance of plasma colloid osmotic pressure in high-flux dialysers. Nephrol Dial Transpl. 2011;26(2):411–3.
Schneditz D, et al. The influence of colloid osmotic pressure on hydrostatic pressures in high- and low-flux hemodialyzers. Artificial Organs. 2018;42(5):525–32.
Baxter, Manufacturer’s Specification Sheet of CT190G Dialyzer. . Available from: https://www.baxter.com/assets/downloads/al09086-cellulosic-brochure-final.pdf.
Sato Y, Mineshima M, Ishimori I, Kaneko I, Akiba T, Teraoka S. Effect of hollow fiber length on solute removal and quantification of internal filtration rate by Doppler ultrasound. Int J Artif Organs. 2003;26(2):129–34.
Ronco C, Brendolan A, Lupi A, Metry G, Levin NW. Effects of a reduced inner diameter of hollow fibers in hemodialyzers. Kidney Int. 2000;58(2):809–17.
Maheshwari V, Thijssen S, Tao X, Fuertinger D, Kappel F, Kotanko P. A novel mathematical model of protein-bound uremic toxin kinetics during hemodialysis. Sci Rep. 2017;7(1):10371.
National Kidney Foundation. KDOQI Clinical Practice Guideline for Hemodialysis Adequacy. update. Am J Kidney Dis, 2015. 2015;66(5):884–930.
Besarab A, Sherman R. The relationship of recirculation to access blood flow. Am J Kidney Dis. 1997;29(2):223–9.
Al-Ghonaim M, et al. Relation between access blood flow and mortality in chronic hemodialysis patients. Clin J Am Soc Nephrol. 2008;3(2):387–91.
Kim TR, Hadidi M, Motevalian SP, Sunohara T, Zydney AL. Effects of plasma proteins on the transport and surface characteristics of polysulfone/polyethersulfone and asymmetric cellulose triacetate high flux dialyzers. Artificial Organs. 2018;42(11):1070–7.
Han, S., et al., Proteomics investigations into serum proteins adsorbed by high-flux and low-flux dialysis membranes. PROTEOMICS – Clinical Applications, 2017. 11(11-12): p. 1700079.
Castro MC, Romao JE Jr, Marcondes M. Measurement of blood urea concentration during haemodialysis is not an accurate method to determine equilibrated post-dialysis urea concentration. Nephrol Dial Transplant. 2001;16(9):1814–7.
Nisha R, et al. Biochemical evaluation of creatinine and urea in patients with renal failure undergoing hemodialysis. J Clin Path Lab Med. 2017;1:1–5.
Acknowledgments
We thank Dr. Belghasem in the Department of Pathology and Laboratory Medicine at Boston University for his contribution to the summary figure. We also thank James K. Goebel in the Engineering Information Technology Office at Boston University for his help in setting up the COMSOL Batch mode.
Funding
This project was funded by National Institutes of Health (NIH) grants R01-HL132325 and R01-CA175382 to VCC, NIH grant T32 HL007224 to OA, and Boston University’s College of Engineering Distinguished Professorship support to JYW.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yu, J., Chitalia, V.C., Akintewe, O.O. et al. Determinants of Hemodialysis Performance:Modeling Fluid and Solute Transport in Hollow-Fiber Dialyzers. Regen. Eng. Transl. Med. 7, 291–300 (2021). https://doi.org/10.1007/s40883-019-00135-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-019-00135-0