Abstract
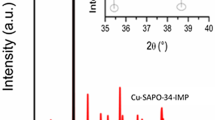
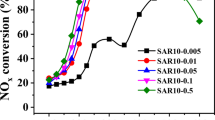
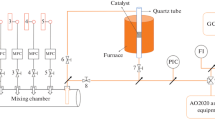
SAPO-34 were synthesized using three structure-directing agents (SDAs), i.e., tetraethylammonium hydroxide (TEAOH), triethylamine (TEA), and morpholine (MO). These SAPO-34 supports were used to prepare Cu/SAPO-34 catalysts via two different Cu-exchange methods: incipient wetness impregnation (IWI) and solid-state ion exchange (SSIE). The catalytic performance of Cu/SAPO-34(TEAOH, TEA, MO) catalysts prepared with IWI and SSIE before and after exposure to water vapor at 70 °C was systemically examined, and their deactivation behavior during low-temperature NH3-SCR reaction was studied. These catalysts were characterized by XRD, BET, ICP-SFMS, SEM/EDX, solid-state NMR, CO-DRIFTS, NO-DRIFTS, and H2-TPR. The various characterization findings for the Cu/SAPO-34 catalysts suggest that the distribution of different Cu2+ species and the mobility of Cu2+ in chabazite (CHA) structure are important for the low-temperature deactivation and regeneration behaviors of the Cu/SAPO-34(TEAOH, TEA, MO)-IWI and -SSIE during the NH3-SCR reaction. Thus, it has been determined that the choice of SDA and Cu-exchange method is vital to design of an efficient Cu/SAPO-34 catalyst that is highly active during a NH3-SCR reaction and has a high tolerance for the low-temperature deactivation caused by exposure to water vapor.







Similar content being viewed by others
References
Fickel, D.W., D’Addio, E., Lauterbach, J.A., Lobo, R.F.: The ammonia selective catalytic reduction activity of copper-exchanged small-pore zeolites. Appl. Catal. B Environ. 102(3-4), 441–448 (2011)
Leistner, K., Mihai, O., Wijayanti, K., Kumar, A., Kamasamudram, K., Currier, N.W., Yezerets, A., Olsson, L.: Comparison of Cu/BEA, Cu/SSZ-13 and Cu/SAPO-34 for ammonia-SCR reactions. Catal. Today. 258, 49–55 (2015)
Hammershoi, P.S., Vennestrom, P.N.R., Falsig, H., Jensen, A.D., Janssens, T.V.W.: Importance of the Cu oxidation state for the SO2-poisoning of a Cu-SAPO-34 catalyst in the NH3-SCR reaction. Appl. Catal. B-Environ. 236, 377–383 (2018)
Wang, D., Jangjou, Y., Liu, Y., Sharma, M.K., Luo, J.Y., Li, J.H., Kamasamudram, K., Epling, W.S.: A comparison of hydrothermal aging effects on NH3-SCR of NOx over Cu-SSZ-13 and Cu-SAPO-34 catalysts. Appl. Catal. B-Environ. 165, 438–445 (2015)
Briend, M., Vomscheid, R., Peltre, M.J., Man, P.P., Barthomeuf, D.: Influence of the choice of the template on the short- and long-term stability of SAPO-34 zeolite. J. Phys. Chem. 99(20), 8270–8276 (1995)
Woo, J., Leistner, K., Bernin, D., Ahari, H., Shost, M., Zammit, M., Olsson, L.: Effect of various structure directing agents (SDAs) on low-temperature deactivation of Cu/SAPO-34 during NH3-SCR reaction. Catal. Sci. Technol. 8(12), 3090–3106 (2018)
Woo, J., Leistner, K., Bernin, D., Ahari, H., Shost, M., Zammit, M., Olsson, L.: Understanding the mechanism of low temperature deactivation of Cu/SAPO-34 exposed to various amount of water vapor in NH3-SCR reaction. Catal. Sci. Technol. 9(14), 3623–3636 (2019)
Woo, J., Bernin, D., Ahari, H., Shost, M., Zammit, M., Olsson, L.: Regeneration of Cu/SAPO-34(MO) with H2O only: too good to be true? Catal. Sci. Technol. 10(5), 1529–1538 (2020)
Woo, J., Bernin, D., Ahari, H., Shost, M., Zammit, M., Olsson, L.: Regeneration of water-deactivated Cu/SAPO-34(MO) with acids. Catal. Sci. Technol. 10(5), 1539–1550 (2020)
Paolucci, C., Khurana, I., Parekh, A.A., Li, S.C., Shih, A.J., Li, H., Di Iorio, J.R., Albarracin-Caballero, J.D., Yezerets, A., Miller, J.T., Delgass, W.N., Ribeiro, F.H., Schneider, W.F., Gounder, R.: Dynamic multinuclear sites formed by mobilized copper ions in NOx selective catalytic reduction. Science. 357(6354), 898–903 (2017)
Gao, F., Walter, E.D., Washton, N.M., Szanyi, J., Peden, C.H.F.: Synthesis and evaluation of Cu/SAPO-34 catalysts for NH3-SCR 2: solid-state ion exchange and one-pot synthesis. Appl. Catal. B Environ. 162, 501–514 (2015)
Wang, D., Gao, F., Peden, C.H.F., Li, J., Kamasamudram, K., Epling, W.S.: Selective catalytic reduction of NOx with NH3 over a Cu-SSZ-13 catalyst prepared by a solid-state ion-exchange method. ChemCatChem. 6(6), 1579–1583 (2014)
Leistner, K., Olsson, L.: Deactivation of Cu/SAPO-34 during low-temperature NH3-SCR. Appl. Catal. B Environ. 165, 192–199 (2015)
Yan, C., Cheng, H., Yuan, Z., Wang, S.: The role of isolated Cu2+ location in structural stability of Cu-modified SAPO-34 in NH3-SCR of NO. Environ. Technol. 36(2), 169–177 (2015)
Fan, S., Xue, J., Yu, T., Fan, D., Hao, T., Shen, M., Li, W.: The effect of synthesis methods on Cu species and active sites over Cu/SAPO-34 for NH3-SCR reaction. Catal. Sci. Technol. 3(9), 2357–2364 (2013)
Wang, L., Li, W., Qi, G., Weng, D.: Location and nature of Cu species in Cu/SAPO-34 for selective catalytic reduction of NO with NH3. J. Catal. 289, 21–29 (2012)
Wang, D., Zhang, L., Li, J., Kamasamudram, K., Epling, W.S.: NH3-SCR over Cu/SAPO-34 – zeolite acidity and Cu structure changes as a function of Cu loading. Catal. Today. 231, 64–74 (2014)
Wang, J., Yu, T., Wang, X.Q., Qi, G.S., Xue, J.J., Shen, M.Q., Li, W.: The influence of silicon on the catalytic properties of Cu/SAPO-34 for NOx reduction by ammonia-SCR. Appl. Catal. B-Environ. 127, 137–147 (2012)
Hadjiivanov, K., Dimitrov, L.: IR spectroscopy study of CO and NOx adsorption on a Cu/Zr-HMS catalyst. Microporous Mesoporous Mater. 27(1), 49–56 (1999)
Hadjiivanov, K.I., Kantcheva, M.M., Klissurski, D.G.: IR study of CO adsorption on Cu-ZSM-5 and CuO/SiO2 catalysts: [sigma] and [small pi] components of the Cu+-CO bond. J. Chem. Soc. Faraday Trans. 92(22), 4595–4600 (1996)
Szanyi, J., Kwak, J.H., Zhu, H., Peden, C.H.F.: Characterization of Cu-SSZ-13 NH3 SCR catalysts: an in situ FTIR study. Phys. Chem. Chem. Phys. 15(7), 2368–2380 (2013)
Pieplu, T., Poignant, F., Vallet, A., Saussey, J., Lavalley, J.C., Mabilon, J.: Oxidation state of copper during the reduction of NOx with propane on H-Cu-ZSM-5 in excess oxygen. Stud. Surf. Sci. Catal. 96, 619–629 (1995)
Palomino, G.T., Bordiga, S., Zecchina, A., Marra, G.L., Lamberti, C.: XRD, XAS, and IR characterization of copper-exchanged Y zeolite. J. Phys. Chem. B. 104(36), 8641–8651 (2000)
Zheng, Q., Ying, L., Shouying, H., Pengzhen, C., Xinbin, M.: Clarification of copper species over Cu-SAPO-34 catalyst by DRIFTS and DFT study of CO adsorption. SCIENCE CHINA Chem. 60, 912 (2017)
Hun Kwak, J., Zhu, H., Lee, J.H., Peden, C.H.F., Szanyi, J.: Two different cationic positions in Cu-SSZ-13? Chem. Commun. 48(39), 4758–4760 (2012)
Beale, A.M., Gao, F., Lezcano-Gonzalez, I., Peden, C.H.F., Szanyi, J.: Recent advances in automotive catalysis for NOx emission control by small-pore microporous materials. Chem. Soc. Rev. 44(20), 7371–7405 (2015)
Akolekar, D.B., Bhargava, S.K., Foger, K.: FTIR investigations of the adsorption and disproportionation of NO on Cu-exchanged silicoaluminophosphate of type 34. J. Chem. Soc. Faraday Trans. 94(1), 155–160 (1998)
Zhang, R., McEwen, J.-S., Kollár, M., Gao, F., Wang, Y., Szanyi, J., Peden, C.H.F.: NO chemisorption on Cu/SSZ-13: a comparative study from infrared spectroscopy and DFT calculations. ACS Catal. 4(11), 4093–4105 (2014)
Jangjou, Y., Ali, M., Chang, Q., Wang, D., Li, J., Kumar, A., Epling, W.S.: Effect of SO2 on NH3 oxidation over a Cu-SAPO-34 SCR catalyst. Catal. Sci. Technol. 6(8), 2679–2685 (2016)
Paolucci, C., Parekh, A.A., Khurana, I., Di Iorio, J.R., Li, H., Albarracin Caballero, J.D., Shih, A.J., Anggara, T., Delgass, W.N., Miller, J.T., Ribeiro, F.H., Gounder, R., Schneider, W.F.: Catalysis in a cage: condition-dependent speciation and dynamics of exchanged Cu Cations in SSZ-13 zeolites. J. Am. Chem. Soc. 138(18), 6028–6048 (2016)
Berthomieu, D., Delahay, G.: Recent advances in CuI/IIY: experiments and modeling. Catal. Rev. 48(3), 269–313 (2006)
Yu, T., Fan, D., Hao, T., Wang, J., Shen, M., Li, W.: The effect of various templates on the NH3-SCR activities over Cu/SAPO-34 catalysts. Chem. Eng. J. 243, 159–168 (2014)
Gao, F., Walter, E.D., Washton, N.M., Szanyi, J., Peden, C.H.F.: Synthesis and evaluation of Cu-SAPO-34 catalysts for ammonia selective catalytic reduction. 1. Aqueous solution ion exchange. ACS Catal. 3(9), 2083–2093 (2013)
Deka, U., Juhin, A., Eilertsen, E.A., Emerich, H., Green, M.A., Korhonen, S.T., Weckhuysen, B.M., Beale, A.M.: Confirmation of isolated Cu2+ ions in SSZ-13 zeolite as active sites in NH3-selective catalytic reduction. J. Phys. Chem. C. 116(7), 4809–4818 (2012)
Wang, J., Fan, D., Yu, T., Wang, J., Hao, T., Hu, X., Shen, M., Li, W.: Improvement of low-temperature hydrothermal stability of Cu/SAPO-34 catalysts by Cu2+ species. J. Catal. 322, 84–90 (2015)
Xiang, X., Wu, P., Cao, Y., Cao, L., Wang, Q., Xu, S., Tian, P., Liu, Z.: Investigation of low-temperature hydrothermal stability of Cu-SAPO-34 for selective catalytic reduction of NOx with NH3. Chin. J. Catal. 38(5), 918–927 (2017)
Lomachenko, K.A., Borfecchia, E., Negri, C., Berlier, G., Lamberti, C., Beato, P., Falsig, H., Bordiga, S.: The Cu-CHA deNOx catalyst in action: temperature-dependent NH3-assisted selective catalytic reduction monitored by operando XAS and XES. J. Am. Chem. Soc. 138(37), 12025–12028 (2016)
Gao, F., Mei, D., Wang, Y., Szanyi, J., Peden, C.H.F.: Selective catalytic reduction over Cu/SSZ-13: linking homo- and heterogeneous catalysis. J. Am. Chem. Soc. 139(13), 4935–4942 (2017)
Acknowledgments
We acknowledge the Chalmers Materials Analysis Laboratory, Chalmers University of Technology, for the use of the SEM instrument and for the support from staff. The Swedish NMR Centre is gratefully acknowledged for spectrometer time.
Funding
We gratefully acknowledge FCA USA LLC and the Swedish Research Council (642-2014-5733) for their funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(PDF 1502 kb)
Rights and permissions
About this article
Cite this article
Woo, J., Wang, A., Bernin, D. et al. Impact of Different Synthesis Methods on the Low-Temperature Deactivation of Cu/SAPO-34 for NH3-SCR Reaction. Emiss. Control Sci. Technol. 7, 198–209 (2021). https://doi.org/10.1007/s40825-020-00182-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40825-020-00182-y