Abstract
Present investigation focuses on the fractional conversion of low volatile weakly caking coal (LVWC) under the standard set of operating conditions for gasification. For this purpose, samples of LVWC of different ash content have been collected from Kusunda Area of Bharat Coking Coal Ltd. Gasification results were validated using Homogenous and Shrinking Core kinetic models and the suitability of selected samples for gasification was assessed by estimating the activation energy. The values of activation energy for LVWC samples were obtained in the range of 25.17–44.09 kJ/mol. Further, empirical models were developed to correlate the response of interest with the input variables (temperature, residence time and CO2 flow rate). The significance of these developed empirical models was checked using analysis of variance (ANOVA).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Among renewable and non-renewable energy resources, fossil fuel is expected to maintain its contribution of 79% in the energy consumption even in 2050 besides 81% at present (IEEJ 2019). Many countries are reducing their dependence on fossil fuels due to the adverse impact of emissions associated with it on the climate. Coal is the most abundant fossil fuel as compared to oil and gas used by several sectors such as power generation, cement, manufacturing, iron, and steel industries. However, developing nations like India and ASEAN will be more dependent on coal by 2050 than today as coal is the only fuel which has the potential to meet the energy needs of these countries, 82% of overall coal consumption will be rigorous in Asia by 2050 (IEEJ 2019). Researchers are more focused over the clean utilization of coal due to continuous increasing demand of coal as a fuel. Combustion of coal suffer from several disadvantages for example lower efficiency, ash formation and carbon dioxide emission. Thus, gasification of coal has been considered as a clean and a best approach to convert carbonaceous fuel into syngas. Syngas can be further used for synthetic natural gas, Fisher-Tropsch liquid and other chemicals. Coal gasification can consequently be regarded as Clean Coal Technology (CCT) along with Carbon Capture and Sequestration which emerge as Zero Emission Technology.
The achievable percentage of carbon conversion in coal gasification depends on various parameters such as rank of coal, gasification medium, particle size, residence time, temperature, pressure, mineral matter and many more. Intensive studied have been done on coal gasification kinetics all over the world. A considerable variation in the reactivity of coal has been found, as the rank of the coal changes or even in the samples of the same rank coal. Volatile content and reactivity of char is associated with each other, i.e. reactivity of char increases with increase in volatile matter content (Beamish et al. 1998; Zhang et al. 2006; Kim et al. 2011). Zhang et al. (2006) have reported that lower rank coals are more reactive than high rank coals because lower rank coals have more concentration of active sites in their matrix than higher rank coals. Findings of Zhang et al. (2006) are in good agreement with the other researchers (Kim et al. 2011; Jenkins et al. 1973).
Gasification performance has been greatly affected by the size of coal particle as well, Jayaraman et al. (2015) investigated the effect of particle size on gasification reactivity and reported that initially char conversion rate during gasification is identical for all particle size but with the time, difference in gasification rate has been observed between different particle sizes. Burnout time is shortened and coal burning rate increases with decrease in particle size (Matsui et al. 1987; Liu et al. 2000; Kajitani et al. 2006). Finer coal particles improve the gasification performance because smaller coal particles are favourable to ignition of coal particles.
Effect of gasification temperature has also been investigated by several researchers. Xiao et al. (2006) reported that enhancement in carbon conversion during gasification reaction by oxidation has been observed with increase in bed temperature and almost linear relationship has been shown by Boudourd and water gas shift reaction. Results of Xiao et al. (2006) are in good agreement with the other authors (Kim et al. 2011; Liu et al. 2008). Corella et al. (2006) have reported that high throughput and low tar content in product gases and high gasification temperature (850–900 °C) is required for the high carbon conversion and enhanced gasification rate. But apart from this it has been observed that above 750 °C product gases contain less than 20% by volume CO2, when gasifier operates at atmospheric pressure, therefore there is no CO2 capture. Various kinetic models have been used in literature to carry out gasification kinetic analysis by fitting their experimental results into kinetic models (Ye et al. 1998; Tanner and Bhattacharya 2016; Xu et al. 2019, 2021). Gasification behaviour for variety of Indian coal has been available in the literature (Jayanti et al. 2007; Baruah and Khare 2007; Mahapatro and Mahanta 2020; Chavan et al. 2012; Rajput et al. 2021; Mandapati and Ghodke 2020; Paul et al. 2021) but study on gasification of Low Volatile Weakly Caking coal (LVWC) has not been available in the literature. Therefore, gasification behaviour of LVWC has been studied in the present investigation. In order to identify the interactional effect of different operating parameters on LVWC gasification, empirical models have also been developed.
2 Materials and methods
Low Volatile Weakly Caking coal (LVWC) has been selected for carrying out gasification experiments. Caking property of the LVWC makes it suitable to form coke which has application in steel making industries. The samples of LVWC used in this study have been collected from Kusunda Area of Bharat Coking Coal Ltd., Jharkhand. LVWC has extremely variable composition and physical characteristics. LVWC samples of 36.13% and 42.09% ash have been selected to carry out gasification experiments. Coal samples have been prepared to the size of − 212 µm for carrying out the experiments. Five grams of each sample has been taken to perform gasification experiments. Some preliminary tests have been carried out to eliminate the effect of sample size and sample amount. Coal samples are subjected to proximate and ultimate analysis, the obtained results are tabulated in Tables 1 and 2, respectively. Calculated values of hardgrove grinding index (HGI), low temperature grey king assay (LTGK), caking index of LVWC have also been reported in Table 3.
3 Experimental procedure
A vertical tube furnace has been used to carry out gasification experiments in the present investigation. Schematic diagram of vertical tube furnace is shown in Fig. 1. It is a type of thermogravimetric analyser (TGA). Thermogravimetric analyser is used for the physical and chemical analysis of coal sample as a function of temperature with constant time or function of time with constant temperature. Usually, weight loss of the coal sample against temperature change is measured in this furnace under controlled conditions. It consists of high precision balance and sample holder. Precision balance of the apparatus is 0.1 gm. Test samples are kept in the sample holder which is located in the furnace. An inert gas is used to control and purge the environment. An electronic weight balance is attached at the top of the furnace for continuous recording of weight loss. Continuous recording of the weight loss by gradually increase in the temperature was done. Vertical tube furnace has been operated at maximum temperature of 1473 K (1200 °C), 5.3 m3 is the maximum operating capacity of gasification chamber and maximum 5 gm sample can be used for the continuous recording of weight loss. The electronic weighing balance is attached at the top of the furnace.
4 Results and discussion
4.1 Effect of residence time and temperature on fractional gasification
Experiments have been performed to study the progress of the gasification reactions with residence time at different temperatures for the samples of LVWC. The results obtained at five different temperatures (1173, 1223, 1273, 1323 and 1373 K) for different gasification time (5–50 min) have been reported in Table 4. The results show that with the increase in residence time, fractional conversion increases which is because of increase in contact time between coal samples and CO2 gas. Fractional conversion increases with the increase in gasification temperature because higher bed temperature accelerates the chemical reactions between carbon and CO2 gas. Highest degree of fractional conversion has been obtained at 1373 K. Maximum gasification is achieved at 40–50 min. Further increase in residence time has practically no effect on fractional conversion of coal samples.
The results reported in Table 4 at 1373 K have been plotted in Fig. 2. This figure shows slightly higher fractional conversion in LVWC-I. This shows that rate of gasification in case of LVWC- I is slightly higher than LVWC-II. This figure supports our earlier finding that with increase in ash content the fractional conversion decreases. It means higher carbon content results into faster rate of gasification. Higher amount of carbon results into higher concentration of reactant (carbon) in contact with CO2 gas which is the reason for the faster rate of reaction between carbon and CO2. Thus, higher carbon content results into faster rate of gasification. Other temperatures (1173, 1223 and 1273 K) have also shown similar trends. In case of high ash, carbon particles supposed to be locked in the matrix of mineral matter which offers hinderance in the path of CO2 gas penetration into carbon particles whereas CO2 gas easily penetrates into carbon particles in case of low ash coal samples.
In the present investigation, Homogenous model (Eq. (1)) and shrinking core model (Eq. (2)) have been used by considering the fact of simplicity and accuracy of these models to describe experimental data. The results shown in Table 4 have been analysed by using both the models,
where X is carbon fractional conversion, k is rate constant for first order reaction kinetics, kc is rate constant for shrink core model and t is residence time.
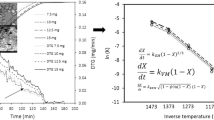
The results of both the modes have been plotted in Figs. 3, 4, 5, 6, which shows straight line relationship throughout the gasification process of LVWC-I and LVWC-II. The obtained experimental results for the samples have been well described by homogenous and shrinking core model. At different temperatures, reaction rate constant k and kc has been calculated by the slop of straight line, which is given in the Table 5.
The obtained values of ln k and ln kc have been plotted against 1/T in Figs. 7 and 8 to estimate the activation energy of gasification for both the coal samples. The well-known Arrhenius equation k = A \({\text{e}}^{{ - \frac{E}{RT}}}\) has been use to estimate activation energy:
where A is pre-exponential factor, E is activation energy, R is universal gas constant (8.314 J/mol K) and T is reaction temperature (K).
The obtained values of activation energy for homogeneous and shrinking core models are in the range of 29.98–44.09 and 25.17–41.26 kJ/mol for LVWC-I and LVWC-II, respectively.
4.2 Full factorial design
The statistical design of experiments has been used to identify the effect of independent variables and their interactional effect in comparatively smaller number of experiments. Mathematical and statistical techniques are combined by Response surface methodology (RSM). Modelling and analysis of the problems in which response of interest is affect by various independent variables can be easily done by such techniques (Tan et al. 2008; Fermoso et al. 2010; Hattingh et al. 2011). In this study, RSM using 2n full factorial design has been used to develop regression equation between the gasification of LWVC and independent variables. The variable parameters and their selected range with actual and coded values have been tabulated in Table 6.
Following first order regression equation has been used to develop empirical models for LVWC:
where X1 is coded form of gasification temperature, X2 is coded form of residence time, X3 is coded form of flow rate.
Relation between actual and coded values of parameters are given as follows:
Average value of gasification results has been estimated by regression coefficient \(a_{o} .\) Effect of gasification temperature, residence time and CO2 flow rate has been shown by regression coefficients a1, a2 and a3, respectively. Interactional effect of two and three variable has been shown by coefficients a12, a23, a13, and a123, respectively.
Design-Expert Software has been used for the regression analysis of experimental data. Results obtained after analysing the individual and interactional effect of variables on response have been interpreted by Analysis of Variance (ANOVA). Significance and consistency of the developed model can be evaluated by three types of test i.e. significance of terms, regression model and lack of fit. Effectiveness of the variables has been determined by significance of terms. Effectives of variables depend on the F-value and probability value (p-value). Probability value describes the proximity of results attainment to the actual experimental results. Effect of variables on response has been identified by F-value. If the variable has smaller p-value (< 0.05) and higher F-value then the variable has considerable effect on the response. Precision of experimental data has been evaluated using regression model which depends on the regression coefficient (R2) and adjusted regression coefficient (Adj-R2) values.
4.3 Empirical model for LVWC gasification
The 23 full factorial design matrix and results showing degree of gasification for the two samples of LVWC are represented in Table 7. The results of ANOVA analysis for the degree of gasification of both the samples of LVWC are shown in Tables 8 and 9.
The empirical model in terms of coded values of process variables using ANNOVA analysis for both the samples of LVWC are as follows:
LVWC-I:
LVWC-II:
p-value and higher F-values of developed models (Eqs. (5) and (6)) for the LVWC-I and LVWC-II have been reported in Tables 8 and 9, respectively. p-values lower than 0.0001 and higher F-values validate the implication of both the models to estimate the gasification behaviour of LVWC-I and LVWC-II. Further, the accuracy and fitting of experimental data in both the models were additionally confirmed by R2 and Adj-R2 values. R2 values for Eqs. (5), (6) were found to be 0.9993 and 0.999 for LVWC-I and LVWC-II, respectively. Adj-R2 values for Eqs. (5), (6) were found to be 0.9983 and 0.998 for LVWC-I and LVWC-II, respectively. R2 and Adj-R2 values for LVWC-I and LVWC-II are close to unity and are in reasonable agreement, which confirms the precision in experiments.
Further, substantial effect is indicated by positive sign and inconsistent effect is indicated by negative sign in Eqs. (5) and (6). It has been observed from the Eq. (5) that effect of gasification temperature has leading and subsequently followed by residence time and CO2 flow rate. In contrast to the gasification of LVWC-I, it has been observed from Eq. (6) that residence time has leading effect on gasification than temperature and flow rate of carbon dioxide for gasification of LVWC-II. The variations in the results of regression Eqs. (5) and (6) have been shown due difference in fixed carbon and ash content in both the samples of LVWC, the effect of ash content and fixed carbon has already been discussed in earlier section. Three-dimensional surface graphs have been plotted in Figs. 9 and 10 for LVWC-I and LVWC-II, respectively to show the interactional effect of gasification operating variables on the degree of gasification. It has been observed from the Figs. 9a and 10a that with the increase in residence time and gasification temperature, rate of gasification has been increased for both the coal samples. Figures 9b and 10b represents the combined effect of flow rate and residence time on gasification i.e. with the increase in flow rate and residence time, rate of gasification has been increased. Similarly, Figs. 9c and 10c have been shown that with the increase in flow rate & gasification temperature, rate of gasification has been increased for both the coal samples.
5 Conclusions
Present investigation on gasification kinetics of LVWC is useful to understand the gasification behaviour of LVWC samples with variable ash content and its suitability for gasification. Fractional carbon conversion during gasification of LVWC increases with increase in residence time and gasification temperature. Gasification rate in case of LVWC-I is slightly higher than that of LVWC-II due to lower ash content in LVWC-I than that of LVWC-II. Homogenous model and Shrinking Core model validated the results obtained for LVWC gasification. Activation energy for gasification of LVWC samples are in the range of 25.17–44.09 kJ/mol. Empirical model of LVWC-I indicates gasification temperature has upmost effect on gasification followed by residence time and CO2 flow rate. While empirical model of LVWC-II indicates that residence time has the uppermost effect on gasification followed by temperature and CO2 flow rate.
References
Baruah BP, Khare P (2007) Desulfurization of oxidized Indian coals with solvent extraction and alkali treatment. Energy Fuels 21(4):2156–2164. https://doi.org/10.1021/ef070087a
Beamish BB, Shaw KJ, Rodgers KA, Newman J (1998) Thermogravimetric determination of the carbon dioxide reactivity of char from some New Zealand coals and its association with the inorganic geochemistry of the parent coal. Fuel Process Technol 53(3):243–253. https://doi.org/10.1016/S0378-3820(97)00073-8
Chavan PD, Sharma T, Mall BK, Rajurkar BD, Tambe SS, Sharma BK, Kulkarni BD (2012) Development of data-driven models for fluidized-bed coal gasification process. Fuel 93:44–51. https://doi.org/10.1016/j.fuel.2011.11.039
Corella J, Toledo JM, Molina G (2006) Steam gasification of coal at low−medium (600–800 °C) temperature with simultaneous CO2 capture in fluidized bed at atmospheric pressure: the effect of inorganic species. 1. Literature review and comments. Ind Eng Chem Res 45(18):6137–6146. https://doi.org/10.1021/ie0602658
Fermoso J, Gil MV, Arias B, Plaza MG, Pevida C, Pis JJ, Rubiera F (2010) Application of response surface methodology to assess the combined effect of operating variables on high-pressure coal gasification for H2-rich gas production. Int J Hydrog Energy 35(3):1191–1204. https://doi.org/10.1016/j.ijhydene.2009.11.046
Hattingh BB, Everson RC, Neomagus HW, Bunt JR (2011) Assessing the catalytic effect of coal ash constituents on the CO2 gasification rate of high ash South African coal. Fuel Process Technol 92(10):2048–2054. https://doi.org/10.1016/j.fuproc.2011.06.003
IEEJ Outlook (2019). https://eneken.ieej.or.jp/data/8122.pdf
Jayanti S, Maheswaran K, Saravanan V (2007) Assessment of the effect of high ash content in pulverized coal combustion. Appl Math Model 31(5):934–953. https://doi.org/10.1016/j.apm.2006.03.022
Jayaraman K, Gokalp I, Bonifaci E, Merlo N (2015) Kinetics of steam and CO2 gasification of high ash coal–char produced under various heating rates. Fuel 154:370–379. https://doi.org/10.1016/j.fuel.2015.02.091
Jenkins RG, Nandi SP, Walker PL Jr (1973) Reactivity of heat-treated coals in air at 500 °C. Fuel 52(4):288–293. https://doi.org/10.1016/0016-2361(73)90059-8
Kajitani S, Suzuki N, Ashizawa M, Hara S (2006) CO2 gasification rate analysis of coal char in entrained flow coal gasifier. Fuel 85(2):163–169. https://doi.org/10.1016/j.fuel.2005.07.024
Kim YT, Seo DK, Hwang J (2011) Study of the effect of coal type and particle size on char–CO2 gasification via gas analysis. Energy Fuels 25(11):5044–5054. https://doi.org/10.1021/ef200745x
Liu GS, Tate AG, Bryant GW, Wall TF (2000) Mathematical modeling of coal char reactivity with CO2 at high pressures and temperatures. Fuel 79(10):1145–1154. https://doi.org/10.1016/S0016-2361(99)00274-4
Liu TF, Fang YT, Wang Y (2008) An experimental investigation into the gasification reactivity of chars prepared at high temperatures. Fuel 87(4–5):460–466. https://doi.org/10.1016/j.fuel.2007.06.019
Mahapatro A, Mahanta P (2020) Gasification studies of low-grade Indian coal and biomass in a lab-scale pressurized circulating fluidized bed. Renew Energy 150:1151–1159. https://doi.org/10.1016/j.renene.2019.10.038
Mandapati RN, Ghodke P (2020) Modeling of gasification process of Indian coal in perspective of underground coal gasification (UCG). Environ Dev Sustain 22(7):6171–6186. https://doi.org/10.1007/s10668-019-00469-3
Matsui I, Kunii D, Furusawa T (1987) Study of char gasification by carbon dioxide. 1. Kinetic study by thermogravimetric analysis. Ind Eng Chem Res 26(1):91–95. https://doi.org/10.1021/ie00061a017
Paul TR, Nath H, Chauhan V, Sahoo A (2021) Gasification studies of high ash Indian coals using Aspen plus simulation. Mater Today Proc 46:6149–6155. https://doi.org/10.1016/j.matpr.2020.04.033
Rajput SK, Verma S, Gupta A, Paul AR, Jain A, Haque N (2021) Environmental impact assessment of coal gasification in hydrogen production. IOP Conf Ser Earth Environ Sci 795:012029. https://doi.org/10.1088/1755-1315/795/1/012029
Tan IA, Ahmad AL, Hameed DB (2008) Preparation of activated carbon from coconut husk: optimization study on removal of 2, 4, 6-trichlorophenol using response surface methodology. J Hazard Mater 153(1–2):709–717. https://doi.org/10.1016/j.jhazmat.2007.09.014
Tanner J, Bhattacharya S (2016) Kinetics of CO2 and steam gasification of Victorian brown coal chars. Chem Eng J 285:331–340. https://doi.org/10.1016/j.cej.2015.09.106
Xiao R, Zhang M, Jin B, Huang Y, Zhou H (2006) High-temperature air/steam-blown gasification of coal in a pressurized spout-fluid bed. Energy Fuels 20(2):715–720. https://doi.org/10.1021/ef050233h
Xu J, Zuo H, Wang G, Zhang J, Guo K, Liang W (2019) Gasification mechanism and kinetics analysis of coke using distributed activation energy model (DAEM). Appl Therm Eng 152:605–614. https://doi.org/10.1016/j.applthermaleng.2019.02.104
Xu T, Wu Y, Bhattacharya S (2021) Gasification kinetic modelling of Victorian brown coal chars and validity for entrained flow gasification in CO2. Int J Min Sci Technol 31(3):473–481. https://doi.org/10.1016/j.ijmst.2021.03.001
Ye DP, Agnew JB, Zhang DK (1998) Gasification of a South Australian low-rank coal with carbon dioxide and steam: kinetics and reactivity studies. Fuel 77(11):1209–1219. https://doi.org/10.1016/S0016-2361(98)00014-3
Zhang L, Huang J, Fang Y, Wang Y (2006) Gasification reactivity and kinetics of typical Chinese anthracite chars with steam and CO2. Energy Fuels 20(3):1201–1210. https://doi.org/10.1021/ef050343o
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mishra, A., Gautam, S. & Sharma, T. Gasification kinetic studies of low volatile weakly caking coal. Int J Coal Sci Technol 10, 25 (2023). https://doi.org/10.1007/s40789-023-00587-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40789-023-00587-4