Abstract
Purpose of review
The purpose of this review is to encapsulate past and current research efforts focused on stem cell transplantation strategies to resolve radiation-induced cognitive dysfunction.
Recent Findings
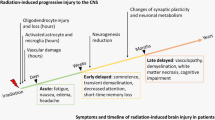
Transplantation of human stem cells in the irradiated brain was first shown to resolve radiation-induced cognitive dysfunction in a landmark paper by Acharya et al., appearing in PNAS in 2009. Since that time, work from the same laboratory as well as other groups have reported on the beneficial (as well as detrimental) effects of stem cell grafting after cranial radiation exposure. Improved learning and memory found many months after engraftment has since been associated with a preservation of host neuronal morphology, a suppression of neuroinflammation, improved myelination, and increased cerebral blood flow. Interestingly, many (if not all) of these beneficial effects can be demonstrated by substituting stem cells with microvesicles derived from human stem cells during transplantation, thereby eliminating many of the more long-standing concerns related to immunorejection and teratoma formation.
Summary
Stem cell and microvesicle transplantation into the irradiated brain of rodents has uncovered some unexpected benefits that hold promise for ameliorating many of adverse neurocognitive complications associated with major cancer treatments. Properly developed, such approaches may provide much needed clinical recourse to millions of cancer survivors suffering from the unintended side effects of their cancer therapies.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lee YW, Cho HJ, Lee WH, Sonntag WE. Whole brain radiation-induced cognitive impairment: pathophysiological mechanisms and therapeutic targets. Biomol Ther (Seoul). 2012;20(4):357–70. https://doi.org/10.4062/biomolther.2012.20.4.357.
Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–89. https://doi.org/10.3322/caac.21349.
Butler JM, Rapp SR, Shaw EG. Managing the cognitive effects of brain tumor radiation therapy. Curr Treat Options in Oncol. 2006;7(6):517–23.
Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24(8):1305–9. https://doi.org/10.1200/JCO.2005.04.6086.
Roman DD, Sperduto PW. Neuropsychological effects of cranial radiation: current knowledge and future directions. Int J Radiat Oncol Biol Phys. 1995;31(4):983–98.
Duffner PK. Long-term effects of radiation therapy on cognitive and endocrine function in children with leukemia and brain tumors. Neurologist. 2004;10(6):293–310. https://doi.org/10.1097/01.nrl.0000144287.35993.96.
Sundgren PC, Cao Y. Brain irradiation: effects on normal brain parenchyma and radiation injury. Neuroimaging Clin N Am. 2009;19(4):657–68. https://doi.org/10.1016/j.nic.2009.08.014.
Greene-Schloesser D, Robbins ME. Radiation-induced cognitive impairment—from bench to bedside. Neuro-Oncology. 2012;14(Suppl 4):iv37–44. https://doi.org/10.1093/neuonc/nos196.
Greene-Schloesser D, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, Chan MD. Radiation-induced brain injury: a review. Front Oncol. 2012;2:73. https://doi.org/10.3389/fonc.2012.00073.
•• Parihar VK, Limoli CL. Cranial irradiation compromises neuronal architecture in the hippocampus. Proc Natl Acad Sci U S A. 2013;110(31):12822–7. https://doi.org/10.1073/pnas.1307301110. This was the first time that cranial irradiation was linked to the long-term structural degradation of granule cell neurons in the hippocampus that involved marked reductions in dendritic complexity and spine density.
Greene-Schloesser D, Moore E, Robbins ME. Molecular pathways: radiation-induced cognitive impairment. Clin Cancer Res. 2013;19(9):2294–300. https://doi.org/10.1158/1078-0432.CCR-11-2903.
Tseng BP, Giedzinski E, Izadi A, Suarez T, Lan ML, Tran KK, et al. Functional consequences of radiation-induced oxidative stress in cultured neural stem cells and the brain exposed to charged particle irradiation. Antioxid Redox Signal. 2014;20(9):1410–22. https://doi.org/10.1089/ars.2012.5134.
Parihar VK, Acharya MM, Roa DE, Bosch O, Christie LA, Limoli CL. Defining functional changes in the brain caused by targeted stereotaxic radiosurgery. Transl Cancer Res. 2014;3(2):124–37. https://doi.org/10.3978/j.issn.2218-676X.2013.06.02.
Parihar VK, Allen BD, Tran KK, Chmielewski NN, Craver BM, Martirosian V, et al. Targeted overexpression of mitochondrial catalase prevents radiation-induced cognitive dysfunction. Antioxid Redox Signal. 2015;22(1):78–91. https://doi.org/10.1089/ars.2014.5929.
Acharya MM, Baulch JE, Lusardi TA, Allen BD, Chmielewski NN, Baddour AA, et al. Adenosine kinase inhibition protects against cranial radiation-induced cognitive dysfunction. Front Mol Neurosci. 2016;9:42. https://doi.org/10.3389/fnmol.2016.00042.
Liu R, Page M, Solheim K, Fox S, Chang SM. Quality of life in adults with brain tumors: current knowledge and future directions. Neuro-Oncology. 2009;11(3):330–9. https://doi.org/10.1215/15228517-2008-093.
Benderitter M, Caviggioli F, Chapel A, Coppes RP, Guha C, Klinger M, et al. Stem cell therapies for the treatment of radiation-induced normal tissue side effects. Antioxid Redox Signal. 2014;21(2):338–55. https://doi.org/10.1089/ars.2013.5652.
Graves PR, Siddiqui F, Anscher MS, Movsas B. Radiation pulmonary toxicity: from mechanisms to management. Semin Radiat Oncol. 2010;20(3):201–7. https://doi.org/10.1016/j.semradonc.2010.01.010.
Tsoutsou PG, Koukourakis MI. Radiation pneumonitis and fibrosis: mechanisms underlying its pathogenesis and implications for future research. Int J Radiat Oncol Biol Phys. 2006;66(5):1281–93. https://doi.org/10.1016/j.ijrobp.2006.08.058.
Xue J, Li X, Lu Y, Gan L, Zhou L, Wang Y, et al. Gene-modified mesenchymal stem cells protect against radiation-induced lung injury. Mol Ther. 2013;21(2):456–65. https://doi.org/10.1038/mt.2012.183.
Kursova LV, Konoplyannikov AG, Pasov VV, Ivanova IN, Poluektova MV, Konoplyannikova OA. Possibilities for the use of autologous mesenchymal stem cells in the therapy of radiation-induced lung injuries. Bull Exp Biol Med. 2009;147(4):542–6.
•• Lombaert IM, Brunsting JF, Wierenga PK, Faber H, Stokman MA, Kok T, et al. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS One. 2008;3(4):e2063. A very important finding demonstrating that targeted stem cell transplantation could be used to offset radiation-induced hypo-salivation.
Nanduri LS, Lombaert IM, van der Zwaag M, Faber H, Brunsting JF, van Os RP, et al. Salisphere derived c-Kit+ cell transplantation restores tissue homeostasis in irradiated salivary gland. Radiother Oncol. 2013;108(3):458–63. https://doi.org/10.1016/j.radonc.2013.05.020.
Coppes RP, Stokman MA. Stem cells and the repair of radiation-induced salivary gland damage. Oral Dis. 2011;17(2):143–53. https://doi.org/10.1111/j.1601-0825.2010.01723.x.
Nanduri LS, Maimets M, Pringle SA, van der Zwaag M, van Os RP, Coppes RP. Regeneration of irradiated salivary glands with stem cell marker expressing cells. Radiother Oncol. 2011;99(3):367–72. https://doi.org/10.1016/j.radonc.2011.05.085.
Porock D, Nikoletti S, Kristjanson L. Management of radiation skin reactions: literature review and clinical application. Plast Surg Nurs. 1999;19(4):185–92. 223; quiz 191-2
Francois S, Mouiseddine M, Mathieu N, Semont A, Monti P, Dudoignon N, et al. Human mesenchymal stem cells favour healing of the cutaneous radiation syndrome in a xenogenic transplant model. Ann Hematol. 2007;86(1):1–8. https://doi.org/10.1007/s00277-006-0166-5.
Huang SP, Huang CH, Shyu JF, Lee HS, Chen SG, Chan JY, et al. Promotion of wound healing using adipose-derived stem cells in radiation ulcer of a rat model. J Biomed Sci. 2013;20:51. https://doi.org/10.1186/1423-0127-20-51.
Ebrahimian TG, Pouzoulet F, Squiban C, Buard V, Andre M, Cousin B, et al. Cell therapy based on adipose tissue-derived stromal cells promotes physiological and pathological wound healing. Arterioscler Thromb Vasc Biol. 2009;29(4):503–10. https://doi.org/10.1161/ATVBAHA.108.178962.
Li N, Zhang L, Li H, Fang B. Human CD34+ cells mobilized by granulocyte colony-stimulating factor ameliorate radiation-induced liver damage in mice. Stem Cell Res Ther. 2010;1(3):22. https://doi.org/10.1186/scrt22.
Gong W, Guo M, Han Z, Wang Y, Yang P, Xu C, et al. Mesenchymal stem cells stimulate intestinal stem cells to repair radiation-induced intestinal injury. Cell Death Dis. 2016;7(9):e2387. https://doi.org/10.1038/cddis.2016.276.
Saha S, Bhanja P, Kabarriti R, Liu L, Alfieri AA, Guha C. Bone marrow stromal cell transplantation mitigates radiation-induced gastrointestinal syndrome in mice. PLoS One. 2011;6(9):e24072. https://doi.org/10.1371/journal.pone.0024072.
Semont A, Francois S, Mouiseddine M, Francois A, Sache A, Frick J, et al. Mesenchymal stem cells increase self-renewal of small intestinal epithelium and accelerate structural recovery after radiation injury. Adv Exp Med Biol. 2006;585:19–30.
Kudo K, Liu Y, Takahashi K, Tarusawa K, Osanai M, Hu DL, et al. Transplantation of mesenchymal stem cells to prevent radiation-induced intestinal injury in mice. J Radiat Res. 2010;51(1):73–9.
Zheng K, Wu W, Yang S, Huang L, Chen J, Gong C, et al. Treatment of radiation-induced acute intestinal injury with bone marrow-derived mesenchymal stem cells. Exp Ther Med. 2016;11(6):2425–31. https://doi.org/10.3892/etm.2016.3248.
Chang PY, Qu YQ, Wang J, Dong LH. The potential of mesenchymal stem cells in the management of radiation enteropathy. Cell Death Dis. 2015;6:e1840. https://doi.org/10.1038/cddis.2015.189.
•• Acharya MM, Martirosian V, Chmielewski NN, Hanna N, Tran KK, Liao AC, et al. Stem cell transplantation reverses chemotherapy-induced cognitive dysfunction. Cancer Res. 2015;75(4):676–86. https://doi.org/10.1158/0008-5472.CAN-14-2237. This was the first study to show that cranial transplantation of human neural stem cells could resolve chemotherapy-induced cognitive dysfunction through a mechanism that involved the preservation of host neuronal morphology and the attenuation of neuroinflammation.
Zickri MB, El Aziz DH, Metwally HG. Histological experimental study on the effect of stem cell therapy on adriamycin induced chemobrain. Int J Stem Cells. 2013;6(2):104–12.
•• Acharya MM, Christie LA, Lan ML, Donovan PJ, Cotman CW, Fike JR, et al. Rescue of radiation-induced cognitive impairment through cranial transplantation of human embryonic stem cells. Proc Natl Acad Sci U S A. 2009;106(45):19150–5. https://doi.org/10.1073/pnas.0909293106. This study provided the first demonstration that human stem cell grafting in the irradiated brain could ameliorate radiation-induced cognitive dysfuncion.
Acharya MM, Christie LA, Lan ML, Giedzinski E, Fike JR, Rosi S, et al. Human neural stem cell transplantation ameliorates radiation-induced cognitive dysfunction. Cancer Res. 2011;71(14):4834–45. https://doi.org/10.1158/0008-5472.CAN-11-0027.
Guzowski J, Setlow B, Wagner E, McGaugh J. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. J Neurosci. 2001;21(14):5089–98.
Guzowski JF, Timlin JA, Roysam B, McNaughton BL, Worley PF, Barnes CA. Mapping behaviorally relevant neural circuits with immediate-early gene expression. Curr Opin Neurobiol. 2005;15(5):599–606. https://doi.org/10.1016/j.conb.2005.08.018.
Acharya MM, Christie LA, Lan ML, Limoli CL. Comparing the functional consequences of human stem cell transplantation in the irradiated rat brain. Cell Transplant. 2013;22(1):55–64. https://doi.org/10.3727/096368912X640565.
Acharya MM, Martirosian V, Christie LA, Limoli CL. Long-term cognitive effects of human stem cell transplantation in the irradiated brain. Int J Radiat Biol. 2014;90(9):816–20. https://doi.org/10.3109/09553002.2014.927934.
• Acharya MM, Rosi S, Jopson T, Limoli CL. Human neural stem cell transplantation provides long-term restoration of neuronal plasticity in the irradiated hippocampus. Cell Transplant. 2015;24(4):691–702. https://doi.org/10.3727/096368914X684600. This study found that human stem cell transplantation provided long-term neurotrophic support in the irradiated host brain, through a mechanism involving the increased expression of the immediate early gene, activity-regulated cytoskeleton-associated protein (Arc).
Acharya MM, Christie LA, Hazel TG, Johe KK, Limoli CL. Transplantation of human fetal-derived neural stem cells improves cognitive function following cranial irradiation. Cell Transplant. 2014;23(10):1255–66. https://doi.org/10.3727/096368913X670200.
Acharya MM, Martirosian V, Christie LA, Riparip L, Strnadel J, Parihar VK, et al. Defining the optimal window for cranial transplantation of human induced pluripotent stem cell-derived cells to ameliorate radiation-induced cognitive impairment. Stem Cell Transl Med. 2015;4(1):74–83. https://doi.org/10.5966/sctm.2014-0063.
•• Baulch JE, Acharya MM, Allen BD, Ru N, Chmielewski NN, Martirosian V, et al. Cranial grafting of stem cell-derived microvesicles improves cognition and reduces neuropathology in the irradiated brain. Proc Natl Acad Sci U S A. 2016;113(17):4836–41. https://doi.org/10.1073/pnas.1521668113. This study demonstrated that cranially transplanted microvesicles derived from human neural stem cells could be used instead of stem cells themselves, for resolving radiation-induced cognitive impairment and associated pathologies.
Franklin RJ, Bayley SA, Blakemore WF. Transplanted CG4 cells (an oligodendrocyte progenitor cell line) survive, migrate, and contribute to repair of areas of demyelination in X-irradiated and damaged spinal cord but not in normal spinal cord. Exp Neurol. 1996;137(2):263–76. https://doi.org/10.1006/exnr.1996.0025.
Hinks GL, Chari DM, O'Leary MT, Zhao C, Keirstead HS, Blakemore WF, et al. Depletion of endogenous oligodendrocyte progenitors rather than increased availability of survival factors is a likely explanation for enhanced survival of transplanted oligodendrocyte progenitors in X-irradiated compared to normal CNS. Neuropathol Appl Neurobiol. 2001;27(1):59–67.
Niranjan A, Fellows W, Stauffer W, Burton EA, Hong CS, Lunsford LD, et al. Survival of transplanted neural progenitor cells enhanced by brain irradiation. J Neurosurg. 2007;107(2):383–91. https://doi.org/10.3171/JNS-07/08/0383.
Marshall GP II, Scott EW, Zheng T, Laywell ED, Steindler DA. Ionizing radiation enhances the engraftment of transplanted in vitro-derived multipotent astrocytic stem cells. Stem Cells. 2005;23(9):1276–85. https://doi.org/10.1634/stemcells.2005-0073.
Rezvani M, Birds DA, Hodges H, Hopewell JW, Milledew K, Wilkinson JH. Modification of radiation myelopathy by the transplantation of neural stem cells in the rat. Radiat Res. 2001;156(4):408–12.
Chari DM, Gilson JM, Franklin RJ, Blakemore WF. Oligodendrocyte progenitor cell (OPC) transplantation is unlikely to offer a means of preventing X-irradiation induced damage in the CNS. Exp Neurol. 2006;198(1):145–53. https://doi.org/10.1016/j.expneurol.2005.11.023.
• Piao J, Major T, Auyeung G, Policarpio E, Menon J, Droms L, et al. Human embryonic stem cell-derived oligodendrocyte progenitors remyelinate the brain and rescue behavioral deficits following radiation. Cell Stem Cell. 2015;16(2):198–210. https://doi.org/10.1016/j.stem.2015.01.004. This study showed that oligoprogenitor cells cranially transplanted after clinically relevant fractionated protocols could resolve learning, memory, and motor deficts through a mechanism involving re-myelination.
• Joo KM, Jin J, Kang BG, Lee SJ, Kim KH, Yang H, et al. Trans-differentiation of neural stem cells: a therapeutic mechanism against the radiation induced brain damage. PLoS One. 2012;7(2):e25936. https://doi.org/10.1371/journal.pone.0025936. This study showed that neural stem cells injected systemically could trans-differentiate into endothelial cells in the irradiate brain and improve cerebral blood flow.
Belkind-Gerson J, Hotta R, Whalen M, Nayyar N, Nagy N, Cheng L, et al. Engraftment of enteric neural progenitor cells into the injured adult brain. BMC Neurosci. 2016;17:5. https://doi.org/10.1186/s12868-016-0238-y.
Osman AM, Zhou K, Zhu C, Blomgren K. Transplantation of enteric neural stem/progenitor cells into the irradiated young mouse hippocampus. Cell Transplant. 2014;23(12):1657–71. https://doi.org/10.3727/096368913X674648.
Sato Y, Shinjyo N, Sato M, Osato K, Zhu C, Pekna M, et al. Grafting of neural stem and progenitor cells to the hippocampus of young, irradiated mice causes gliosis and disrupts the granule cell layer. Cell Death Dis. 2013;4:e591. https://doi.org/10.1038/cddis.2013.92.
Acknowledgments
Training grant T32 NS082174 (SMS) and grants from the NINDS 5R01 NS074388 (CLL) and from the Defense Threat Reduction Agency HDTRA 1-13-1-0022 (CLL) supported this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Sarah M. Smith and Charles L. Limoli declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Additional information
This article is part of the Topical Collection on Radiation Biology and Stem Cells
Rights and permissions
About this article
Cite this article
Smith, S.M., Limoli, C.L. Stem Cell Therapies for the Resolution of Radiation Injury to the Brain. Curr Stem Cell Rep 3, 342–347 (2017). https://doi.org/10.1007/s40778-017-0105-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40778-017-0105-5