Abstract
Introduction
Here we examine the relationship between achieving different levels of disease activity with tofacitinib (an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis), long-term structural progression, and patient-reported physical function.
Methods
This was a post hoc analysis of two 24-month, phase III randomized controlled trials in methotrexate (MTX)-naïve (ORAL Start [NCT01039688]) or MTX-inadequate responder (IR) patients (ORAL Scan [NCT00847613]) receiving tofacitinib 5 or 10 mg twice daily as either monotherapy or with background MTX. The modified total Sharp score (mTSS) and Health Assessment Questionnaire-Disability Index (HAQ-DI) were analyzed at month 24 according to disease activity at month 6 defined by the Clinical Disease Activity Index (CDAI) or the Disease Activity Score in 28 joints, C-reactive protein (DAS28CRP).
Results
Mean changes from baseline in mTSS at month 24 were less in patients with CDAI remission at month 6 than in those with CDAI moderate/high disease activity (MDA/HDA) at month 6. A DAS28CRP of < 1.9 most closely approximated CDAI remission (≤ 2.8). Tofacitinib appeared to inhibit joint damage in the presence of persistent inflammation compared with MTX. More patients receiving tofacitinib or MTX with CDAI remission or low disease activity (LDA) at month 6 reported normative HAQ-DI scores (< 0.5) at month 24 than did those with CDAI MDA/HDA.
Conclusion
Regardless of treatment, in both MTX-naïve and MTX-IR patients, remission or LDA at month 6 was associated with successful long-term outcomes: inhibition of structural progression and normative HAQ-DI scores. Long-term outcomes were similar when patients achieved CDAI remission or a DAS28CRP of < 1.9, confirming that this is an appropriate cut-off for remission with DAS28CRP. Tofacitinib potentially inhibits joint damage even with persistent inflammation.
Funding
Pfizer Inc.
Trial registration
Clinicaltrials.gov identifiers: NCT01039688 and NCT00847613.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by inflammation, pain, joint stiffness, and progressive joint destruction [1]. Patients with RA often experience impaired health-related quality of life as a result of symptoms of pain and fatigue, and loss of physical function [2, 3]. Early intervention with disease-modifying antirheumatic drugs (DMARDs) can reduce or reverse signs and symptoms of disease, inhibit progression of structural damage, and form a cornerstone of treatment [4, 5].
Tofacitinib is an oral Janus kinase inhibitor for the treatment of RA. The efficacy and safety of tofacitinib administered at doses of 5 and 10 mg twice daily (BID) either as monotherapy or in combination with conventional synthetic DMARDs, mainly methotrexate (MTX), in patients with moderately to severely active RA, have been demonstrated in phase II [6,7,8,9,10,11,12] and phase III [13,14,15,16,17,18,19,20,21,22] randomized controlled trials (RCTs) of up to 24 months’ duration and in long-term extension studies with up to 105 months’ observation [23, 24].
ORAL Start (NCT01039688; trial registered December 23, 2009) [18] and ORAL Scan (NCT00847613; trial registered February 17, 2009) [16] are two phase III RCTs that included radiographic outcomes. ORAL Start was conducted in MTX-naïve patients and ORAL Scan in patients who were MTX-inadequate responders (IR) [16, 18]. Patients received tofacitinib 5 or 10 mg BID as monotherapy versus MTX monotherapy (ORAL Start) or with background MTX versus placebo (ORAL Scan).
In both 24-month trials, tofacitinib improved signs and symptoms of RA and patient-reported outcomes and inhibited progression of structural damage versus comparator treatment by month 6 [16, 18, 19]. However, mean changes in van der Heijde modified total Sharp scores (mTSS) were statistically significant only with tofacitinib 10 mg BID versus placebo (tofacitinib 5 mg BID vs. placebo P = 0.079) in ORAL Scan [16].
Here we present a post hoc analysis of data from ORAL Start and ORAL Scan which examined the efficacy of tofacitinib (vs. MTX) on long-term radiographic and Health Assessment Questionnaire-Disability Index (HAQ-DI) scores, with a particular focus on the relationship between disease activity levels at month 6 and longer-term outcomes.
Methods
Study Design and Patients
Details of ORAL Start and ORAL Scan have been previously published [16, 18]. Eligible patients were aged ≥ 18 years, fulfilled the American College of Rheumatology 1987 revised criteria [25], and had active disease (≥ 6 tender or painful joints and ≥ 6 swollen joints, plus ≥ 3 distinct joint erosions, or were rheumatoid factor or anti-citrullinated antibody protein positive).
In ORAL Start, MTX-naïve patients were randomized 2:2:1 to tofacitinib 5 or 10 mg BID, or MTX starting at 10 mg per week and increasing to 20 mg per week by week 8. In ORAL Scan, MTX-IR patients were randomized 4:4:1:1 to tofacitinib 5 or 10 mg BID, placebo advanced to tofacitinib 5 mg BID, or placebo advanced to tofacitinib 10 mg BID, all with stable background MTX. Patients receiving placebo were advanced in a blinded manner to tofacitinib if they had not achieved ≥ 20% improvement in swollen and tender joint counts after 3 months (defined as non-responders); after 6 months, all remaining placebo patients were advanced to tofacitinib.
Both studies were conducted in accordance with the Declaration of Helsinki and International Conference on Harmonisation Guidelines for Good Clinical Practice and were approved by the Institutional Review Board at each study center. All patients provided written informed consent. This report presents post hoc analyses of data from two previously published studies [16, 18], and additional ethics approval was not required for these analyses.
Data Analyses and Assessments
In this post hoc analysis, patients receiving tofacitinib or MTX monotherapy in ORAL Start, or tofacitinib + MTX in ORAL Scan, were analyzed according to disease activity at month 6, which was defined by the Clinical Disease Activity Index (CDAI), Disease Activity Score in 28 joints, C-reactive protein (DAS28CRP), or the DAS28–erythocyte sedimentation rate (DAS28ESR). Cut-offs utilized in this analysis using CDAI were: ≤ 2.8 (remission); > 2.8 to ≤ 10 (low disease activity [LDA]); > 10 (moderate/high disease activity [MDA/HDA]). DAS28CRP and DAS28ESR data were analyzed using the traditional cut-offs for remission, LDA, and MDA/HDA (< 2.6; ≥ 2.6 to ≤ 3.2; > 3.2, respectively), and DAS28CRP data were also analyzed using a more stringent cut-off for remission (< 1.9). This stringent cut-off was chosen based on a recent publication examining DAS28CRP disease remission and LDA cut-offs [26]. All analyses are based on the full analysis set (randomized and received ≥ 1 dose of study treatment) and each type of stratification (CDAI and DAS28CRP and DAS28ESR status) defined by its respective non-missing disease activity measure at month 6. Patients randomized to placebo in ORAL Scan were excluded as they advanced to tofacitinib at months 3 or 6, and only one placebo patient achieved remission at month 6.
Radiographic progression (changes from baseline in mTSS) and physical function (HAQ-DI scores) at month 24 were analyzed according to CDAI or DAS28CRP scores at month 6. Radiographic non-progression was defined as ≤ 0 change from baseline in mTSS (non-prespecified endpoint). A normative HAQ-DI score was defined as < 0.5.
This was an exploratory analysis; no formal statistical analyses were prespecified. Categorical endpoints were summarized by frequency (n, %). Continuous efficacy endpoints were summarized using descriptive statistics, including mean and 95% confidence intervals (CI).
The data analysis for this study was performed using SAS software, version 9.2 of the SAS System for Unix. (Copyright© 2008 SAS Institute Inc., Cary, NC, USA). All other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc.
Results
Patients
Overall, 956 patients in ORAL Start and 797 in ORAL Scan were randomized and treated. At month 6, CDAI and DAS28CRP data were available for 1419 and 1420 patients (from both studies), respectively, all of whom were eligible for inclusion in this post hoc analysis. Baseline patient demographics and disease characteristics within the month 6 disease activity subgroups were generally similar across treatment groups (Electronic Supplementary Material Tables S1 and S2). However, in both RCTs, patients with CDAI-defined MDA/HDA at month 6 had higher baseline disease activity and mTSS than did those with LDA (without remission) or remission at month 6. Similarly, baseline disease activity was higher among patients with worse disease status at month 6 when defined by DAS28CRP (data not shown). Across disease activity groups, mean baseline mTSS were higher among patients in ORAL Scan than in ORAL Start.
Relationship Between Disease Activity at Month 6 and Month 24
The proportions of MTX-naïve patients in CDAI remission or with LDA (without remission) at month 6 were higher in those receiving both doses of tofacitinib than in those on MTX in ORAL Start, and they were generally higher in patients in ORAL Start than in those in ORAL Scan (Table 1). Tofacitinib treatment was also associated with lower levels of disease activity at month 6, compared with MTX, when activity was measured by the DAS28CRP. More patients achieved a DAS28CRP of < 2.6 than CDAI remission (≤ 2.8), while the DAS28CRP < 1.9 cut-off yielded similar proportions of patients to those in CDAI remission.
Relationship Between Disease Activity and Long-Term Radiographic Progression
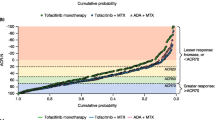
Among patients receiving MTX monotherapy, mean change from baseline in mTSS at month 24 was smaller for patients with CDAI remission at month 6 than for patients with CDAI MDA/HDA at month 6 (Fig. 1a). A similar relationship was evident for patients receiving tofacitinib 5 and 10 mg BID with or without background MTX. However, compared with MTX, less radiographic progression at month 24 was present with tofacitinib treatment in patients with MDA/HDA at month 6 (Fig. 1a).
Mean change from baseline in van der Heijde mTSS at month 24 according to response at month 6 defined by CDAI (a) and CDAI remission, a DAS28CRP of < 1.9, and DAS28CRP of < 2.6 (b). BID twice daily, CDAI Clinical Disease Activity Index, CI confidence interval, DAS28CRP Disease Activity Score in 28 joints, C-reactive protein, IR inadequate responder, LDA low disease activity, MDA/HDA moderate/high disease activity, mTSS modified total Sharp score, MTX methotrexate
For patients enrolled in ORAL Start, larger changes from baseline at month 24 in mTSS were observed in the DAS28CRP < 2.6 category than in the category with CDAI-defined remission at month 6 (Fig. 1b). Patients with a DAS28CRP of < 1.9 at month 6 generally had similar levels of changes in mTSS to those in the CDAI-defined remission category (Fig. 1b), with the exception of patients treated with tofacitinib 10 mg BID in ORAL Scan.
Irrespective of treatment, a higher number of patients in CDAI remission or LDA (without remission) at month 6 were radiographic non-progressors at month 24 (vs. CDAI MDA/HDA), although 95% CI overlapped (Fig. 2a). A higher proportion of patients in the CDAI MDA/HDA category at month 6 were radiographic non-progressors at month 24 with tofacitinib monotherapy than with MTX. In general, a DAS28CRP of < 1.9 provided the best approximation to CDAI remission in terms of radiographic non-progression (Fig. 2b).
Proportion of radiographic non-progressors at month 24 according to disease activity at month 6 defined by CDAI (a) and CDAI remission, DAS28CRP < 1.9, and DAS28CRP < 2.6 (b). Radiographic non-progression is defined as a change in mTSS of ≤ 0.0. BID twice daily, CDAI Clinical Disease Activity Index, CI confidence interval, DAS28CRP Disease Activity Score in 28 joints, C-reactive protein, IR inadequate responder, LDA low disease activity, MDA/HDA moderate/high disease activity, mTSS modified total Sharp score, MTX methotrexate
Relationship Between Disease Activity and Physical Function
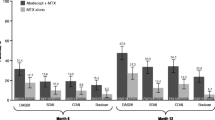
Across treatment groups, there were more patients with CDAI-defined remission at month 6 or LDA who reported normative HAQ-DI scores (< 0.5) at month 24 than there were patients with CDAI MDA/HDA (Fig. 3a).
Proportion of patients reporting normative HAQ-DI scores (< 0.5) at month 24 according to disease activity at month 6 defined by CDAI (a) and CDAI remission, DAS28CRP < 1.9, and DAS28CRP < 2.6 (b). Normative HAQ-DI was defined as a score of < 0.5. BID twice daily, CDAI Clinical Disease Activity Index, CI confidence interval, DAS28CRP Disease Activity Score in 28 joints, C-reactive protein, HAQ-DI Health Assessment Questionnaire-Disability Index, IR inadequate responder, LDA low disease activity, MDA/HDA moderate/high disease activity, MTX methotrexate
In ORAL Start, more patients who received tofacitinib monotherapy reported normative HAQ-DI scores at month 24 than did MTX-treated patients at month 6 with the same level of disease activity, including those with CDAI MDA/HDA at month 6, although the 95% CI overlapped (Fig. 3a). More MTX-naïve than MTX-IR patients reported normative HAQ-DI scores.
Tofacitinib patients with a DAS28CRP of < 2.6 at month 6 reported fewer normative HAQ-DI scores than did patients in CDAI remission and with a DAS28CRP of < 1.9, with the exception of patients in ORAL Scan who received tofacitinib 10 mg (Fig. 3b).
Overall, more patients who achieved CDAI-defined remission or LDA (without remission) at month 6 with tofacitinib monotherapy or with background MTX reported larger improvements from baseline in HAQ-DI scores at month 24 than did those with MDA/HDA (Fig. 4a). Tofacitinib monotherapy was associated with numerically larger improvements in HAQ-DI scores compared with MTX within response subgroups, although the 95% CI overlapped. Improvements reported by patients with CDAI MDA/HDA at month 6 were similar with tofacitinib 5 mg BID, tofacitinib 10 mg BID, and MTX. In general, a DAS28CRP of < 1.9 best approximated CDAI remission in terms of changes in HAQ-DI scores (Fig. 4b).
Mean change from baseline at month 24 in HAQ-DI score according to disease activity at month 6 defined by CDAI (a) and CDAI remission, DAS28CRP < 1.9, and DAS28CRP < 2.6 (b). BID twice daily, CDAI Clinical Disease Activity Index, CI confidence interval, DAS28CRP Disease Activity Score in 28 joints, C-reactive protein, HAQ-DI Health Assessment Questionnaire-Disability Index, IR inadequate responder, LDA low disease activity, MDA/HDA moderate/high disease activity, MTX methotrexate
Discussion
This post hoc, exploratory analysis of data from two phase III RCTs (ORAL Start and ORAL Scan) evaluated the efficacy of tofacitinib on long-term radiographic outcomes and physical function, based on disease activity at month 6, in MTX-naïve and MTX-IR patients. Data from both RCTs were included in the analysis to explore the impact of prior MTX failure on outcomes.
CDAI-defined remission or LDA (without remission) at month 6 was associated with higher rates of radiographic non-progression at month 24 compared with MDA/HDA at month 6. This indicates that early suppression of inflammation is associated with long-term structural benefit. Compared with MTX, tofacitinib appeared to potentially inhibit joint damage even in the presence of persistent inflammation.
In this analysis, attainment of normative HAQ-DI scores and improvements in HAQ-DI from baseline at month 24 were greater in tofacitinib- or MTX-treated patients who attained remission or LDA (without remission) at month 6 than in those with MDA/HDA. This may be a direct consequence of lower disease activity or could indicate that inhibition of radiographic progression has the potential to translate into important benefits in physical function.
Comparison of outcomes from ORAL Start and ORAL Scan indicated that MTX-naïve patients treated with tofacitinib monotherapy reported greater improvement in HAQ-DI scores than MTX-IR patients receiving tofacitinib with background MTX. MTX-IR patients who did not achieve remission or LDA at month 6 (had MDA/HDA) appeared to be the most difficult to treat in terms of inhibiting radiographic progression and achieving important improvements in physical function by month 24, indicating that patients with already established RA may have irreversible damage and disability. It should be noted that these patients also had higher mTSS at baseline.
The relationship between disease state and subsequent long-term outcomes was generally similar whether activity at month 6 was defined by the CDAI or by the DAS28CRP. However, the choice of cut-offs to define disease states appears to be important. A DAS score of < 2.6 has been traditionally used to define remission, whether using CRP or ESR as inflammatory markers [27]. In our analysis, patients achieved a DAS28CRP of < 2.6 but still had residual disease activity, which yielded uncertainty in predicting long-term outcomes when it was used versus CDAI-defined remission.
In line with our findings, a DAS28CRP of < 2.6 has been previously noted to be a less conservative measure of remission among tofacitinib-treated patients, with CDAI-defined remission rates being closer to Boolean-based remission rates [28]; other cut-offs for DAS28CRP remission and LDA have been proposed [26]. DAS28CRP has also been reported to result in higher remission rates than DAS28ESR when a score of < 2.6 is used for both [26]. When ORAL Start and ORAL Scan data were analyzed by more stringent DAS28CRP cut-offs, a DAS28CRP of < 1.9 consistently produced results most similar to CDAI-defined remission. A DAS28CRP of < 1.9 was also found to be similar to CDAI remission when analyzing data from etanercept clinical trials [26]. The data from ORAL Start and ORAL Scan were also analyzed by DAS28ESR-defined disease status (data not presented here), and when remission was defined as a DAS28ESR of < 2.6, findings were more similar to CDAI remission and a DAS28CRP of < 1.9 than a DAS28CRP of < 2.6.
Potential limitations of the present analysis include the post hoc exploratory nature of this evaluation. Patient numbers in some of the subgroups were small, potentially resulting in higher data variability and, therefore, in wide 95% CI. In addition, the comparability of the level of disease activity across the disease activity groups cannot be confirmed.
Conclusions
Overall, attainment of remission or LDA at month 6, regardless of treatment, provides the best probability of long-term inhibition of radiographic progression of structural damage and important improvements in physical function. However, treatment with tofacitinib resulted in reduced structural progression at month 24 compared with treatment with MTX, even in patients with MDA/HDA at month 6, indicating a partial dissociation between inflammatory disease and joint damage in patients treated with tofacitinib. Long-term outcomes were similar when patients achieved CDAI remission or a DAS28CRP of < 1.9, confirming that this should be the new remission cut-off when utilizing DAS28CRP in clinical practice.
References
Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–38.
Strand V, Singh JA. Newer biological agents in rheumatoid arthritis: impact on health-related quality of life and productivity. Drugs. 2010;70(2):121–45.
Strand V, Khanna D. The impact of rheumatoid arthritis and treatment on patients’ lives. Clin Exp Rheumatol. 2010;28[3 Suppl 59]:S32–40.
Singh JA, Saag KG, Bridges SL Jr, Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68(1):1–26.
Smolen JS, Landewé R, Breedveld FC, Buch M, Burmester G, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73(3):492–509.
Kremer JM, Bloom BJ, Breedveld FC, Coombs JH, Fletcher MP, Gruben D, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 2009;60(7):1895–905.
Tanaka Y, Suzuki M, Nakamura H, Toyoizumi S, Zwillich SH, Investigators TS. Phase II study of tofacitinib (CP-690,550) combined with methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Care Res (Hoboken). 2011;63(8):1150–8.
Fleischmann R, Cutolo M, Genovese MC, Lee EB, Kanik KS, Sadis S, et al. Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis Rheum. 2012;64(3):617–29.
Kremer JM, Cohen S, Wilkinson BE, Connell CA, French JL, Gomez-Reino J, et al. A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum. 2012;64(4):970–81.
Tanaka Y, Takeuchi T, Yamanaka H, Nakamura H, Toyoizumi S, Zwillich S. Efficacy and safety of tofacitinib as monotherapy in Japanese patients with active rheumatoid arthritis: a 12-week, randomized, phase 2 study. Mod Rheumatol. 2015;25(4):514–21.
Coombs JH, Bloom BJ, Breedveld FC, Fletcher MP, Gruben D, Kremer JM, et al. Improved pain, physical functioning and health status in patients with rheumatoid arthritis treated with CP-690,550, an orally active Janus kinase (JAK) inhibitor: results from a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2010;69:413–6.
Wallenstein GV, Kanik KS, Wilkinson B, Cohen S, Cutolo M, Fleishmann R, et al. Effects of the oral Janus kinase inhibitor tofacitinib on patient-reported outcomes in patients with active rheumatoid arthritis: results of two Phase 2 randomised controlled trials. Clin Exp Rheumatol. 2016;34(3):430–42.
van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, García Meijide JA, Wagner S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367(6):508–19.
Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012;367(6):495–507.
Burmester GR, Blanco R, Charles-Schoeman C, Wollenhaupt J, Zerbini C, Benda B, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet. 2013;381(9865):451–60.
van der Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 2013;65(3):559–70.
Kremer J, Li Z-G, Hall S, Fleischmann R, Genovese M, Martin-Mola E, et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2013;159(4):253–61.
Lee EB, Fleischmann R, Hall S, Wilkinson B, Bradley J, Gruben D, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;370(25):2377–86.
Fleischmann R, Strand V, Wilkinson B, Kwok K, Bananis E. Relationship between clinical and patient-reported outcomes in a phase 3 trial of tofacitinib or MTX in MTX-naïve patients with rheumatoid arthritis. RMD Open. 2016;2(1):e000232.
Strand V, Burmester GR, Zerbini CA, Mebus CA, Zwillich SH, Gruben D, et al. Tofacitinib with methotrexate in third-line treatment of patients with active rheumatoid arthritis: patient-reported outcomes from a phase III trial. Arthritis Care Res (Hoboken). 2015;67(4):475–83.
Strand V, Kremer J, Wallenstein G, Kanik KS, Connell C, Gruben D, et al. Effects of tofacitinib monotherapy on patient-reported outcomes in a randomized phase 3 study of patients with active rheumatoid arthritis and inadequate responses to DMARDs. Arthritis Res Ther. 2015;17:307.
Strand V, van Vollenhoven RF, Lee EB, Fleischmann R, Zwillich SH, Gruben D, et al. Tofacitinib or adalimumab versus placebo: patient-reported outcomes from a phase 3 study of active rheumatoid arthritis. Rheumatology (Oxford). 2016;55(6):1031–41.
Wollenhaupt J, Silverfield J, Lee EB, Curtis JR, Wood SP, Soma K, et al. Safety and efficacy of tofacitinib, an oral Janus kinase inhibitor, for the treatment of rheumatoid arthritis in open-label, longterm extension studies. J Rheumatol. 2014;41(5):837–52.
Wollenhaupt J, Silverfield J, Lee EB, Terry K, Kwok K, Abramsky S, et al. Tofacitinib, an oral Janus kinase inhibitor, in the treatment of rheumatoid arthritis: safety and efficacy in open-label, long-term extension studies over 8 years [abstract]. Arthritis Rheumatol 2016;68 (suppl 10). http://acrabstracts.org/abstract/tofacitinib-an-oral-janus-kinase-inhibitor-in-the-treatment-of-rheumatoid-arthritis-safety-and-efficacy-in-open-label-long-term-extension-studies-over-8-years/. Accessed 3 May 2018.
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–24.
Fleischmann R, van der Heijde D, Koenig AS, Pedersen R, Szumski A, Marshall L, et al. How much does Disease Activity Score in 28 joints ESR and CRP calculations underestimate disease activity compared with the Simplified Disease Activity Index? Ann Rheum Dis. 2015;74(6):1132–7.
Fransen J, Creemers MC, van Riel PL. Remission in rheumatoid arthritis: agreement of the disease activity score (DAS28) with the ARA preliminary remission criteria. Rheumatology (Oxford). 2004;43(10):1252–5.
Smolen JS, Aletaha D, Gruben D, Zwillich SH, Krishnaswami S, Mebus C. Remission rates with tofacitinib treatment in rheumatoid arthritis: a comparison of various remission criteria. Arthritis Rheumatol. 2017;69:728–34.
Acknowledgements
We wish to thank all patients who participated in these trials, and investigators and staff of the participating centers.
Funding
These studies were funded by Pfizer Inc. Article processing charges were funded by Pfizer Inc. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing and/or Editorial Assistance
Editorial support under the guidance of the authors was provided by Mark Walker, PhD, at CMC Connect, a division of Complete Medical Communications Ltd, Macclesfield, UK, and Nicole Jones, BSc, on behalf of CMC Connect, and was funded by Pfizer Inc, New York, NY, USA in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med 2015; 163: 461-464).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Disclosures
Vibeke Strand has received grants or research support from and is a consultant for Pfizer Inc. Arthur Kavanaugh has received research support from Pfizer Inc. Alan J. Kivitz is a member of the speakers’ bureau for and has received research grants and consultancy fees from Pfizer Inc. Désirée van der Heijde is a consultant for Pfizer Inc. Josef S. Smolen has received research support from and is a consultant for Pfizer Inc. Ermeg Akylbekova is a consultant for Pfizer Inc from Quintiles Inc. Kenneth Kwok is an employee and shareholder of Pfizer Inc. Arif Soonasra is an employee and shareholder of Pfizer Inc. Mark Snyder is an employee and shareholder of Pfizer Inc. Carol Connell is an employee and shareholder of Pfizer Inc. Eustratios Bananis is an employee and shareholder of Pfizer Inc.
Compliance with Ethics Guidelines
Both studies were conducted in accordance with the Declaration of Helsinki and International Conference on Harmonisation Guidelines for Good Clinical Practice and were approved by the Institutional Review Board at each study center. All patients provided written informed consent. This report presents post hoc analyses of data from two previously published studies [16, 18] and additional ethics approval was not required for these analyses.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.6171377.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Strand, V., Kavanaugh, A., Kivitz, A.J. et al. Long-Term Radiographic and Patient-Reported Outcomes in Patients with Rheumatoid Arthritis Treated with Tofacitinib: ORAL Start and ORAL Scan Post-hoc Analyses. Rheumatol Ther 5, 341–353 (2018). https://doi.org/10.1007/s40744-018-0113-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40744-018-0113-7