Abstract
Viral nephropathy is a term defines glomerular, tubular and/or vascular injury in kidney caused by viruses itself or virus-induced immune mechanisms. It is difficult to prove causality between the renal disease and the viral infection, however, renal biopsy findings can help in this regard. Several viruses such as hepatitis B and C, Human immun deficiciency virus (HIV), Hantavirus, Cytomegalovirus (CMV), an recently Coronavirus are shown to affect the kidney. Treatment of viral nephropathies are unique regarding the diagnosis which can be made only with renal biopsy in most of the situations. We present two patients presented with acute kidney injury and thrombocytopenia caused by different viruses (Hantavirus and HIV) that affect multiple areas in kidney that revealed with kidney biopsy. Supportive treatment in the patient with Hantavirus nephropathy and HIV treatment along with eculizumab and supportive treatment in the patient with HIVAN were successfully implemented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Case presentations
We report two patients who presented with acute kidney injury (AKI) and thrombocytopenia caused by different viruses, revealed through kidney biopsies, that affect multiple compartments in the kidney.
Case 1
A 34-year-old male without any comorbidities presented with fever (39 °C), fatigue and myalgias that had been going on for a week. He reported possible contact with mice in his office. Physical examination was unremarkable. On first admission, blood chemistry revealed mild hepatic enzyme elevation and normal creatinine level [alkaline phosphatase (ALP), 210 U/L; gamma-glutamyl transpeptidase (GGT), 200 U/L; alanine aminotransferase (ALT), 30 U/L; aspartate aminotransferase (AST), 69 U/L; lactate dehydrogenase (LDH), 354 U/L; direct bilirubin, 0.18 mg/dL; indirect bilirubin, 0.53 mg/dL; creatinine 0.97 mg/dL]. His blood count showed thrombocytopenia (30 × 109/L) without any schistocytes on the peripheral blood smear. His creatinine level increased to 2.29 mg/dL in 4 days along with nephrotic range proteinuria (spot urine protein/creatinine, 5,000 mg/g) and hypoalbuminemia (serum albumin, 2.9 g/dL). Viral serologies were sent for hepatitis A, B, and C, HIV, CMV, and Leptospirosis, all coming back negative. ADAMTS 13 activity was 90%, so thrombotic thrombocytopenic purpura (TTP) was excluded. While awaiting the results, his creatinine level continued to go up (3.02 mg/dL) and his thrombocyte level began to increase as well (120 × 109/L). We performed a renal biopsy in order not to miss a diagnosis of cresentic glomerulonephritis (Fig. 1). Serological tests for Hantavirus came back positive. We followed the patient under conservative treatment. Ten days after the first symptoms, his serum creatinine level returned to the normal range (0.94 mg/dL) and proteinuria resolved to normal.
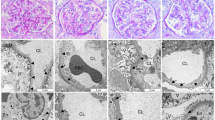
The predominant histological finding of light microscopy examination of the renal biopsy specimen of the patient with Hantavirus nephropathy was abundant interstitial hemorrhage, especially in the medulla (arrow). The integrity of capillaries was lost and there were erythrocyte clusters and fibrin residues. Interstitial lymphocytic infiltration was composed of neutrophils and eosinophils accompanying tubulitis that were evident due to the presence of attacking tubular epithelial cells. The glomerular compartment was unremarkable except for mild mesangial hypercellularity.[Hematoxylin&Eosin (H&E) × 170]. In the immunofluorescence examination of the specimen, there were IgA deposits in the mesangium and minimal C3 deposition in the capillary walls of the glomeruli (not shown). The biopsy was interpreted as acute interstitial nephritis with prominent interstitial hemorrhage which favored Hantavirus nephropathy and the accompanying IgA deposition. Hantaviruses are endotheliotropic viruses and cause hemorrhagic fever with renal syndrome of differing severity. Humans get infected with Hantavirus, which consist of 100,000 new cases every year, through exposure to the feces, urine or other secretions of rodents. Public health programs all around the world therefore, are working arduously on annihilating the root of this problem, i.e. rodents, in all the residential areas [1]. Symptoms and findings such as thrombocytopenia, renal failure and respiratory failure are related to affected small blood vessels [2]. In a renal biopsy, vascular involvement is the main evidence of viral nephropathy and is found as an interstitial hemorrhage mostly in the outer medulla. Inducing an innate and humoral response and causing mesangial immune complex deposition (mostly C3 and IgM) were suggested as responsible mechanisms for Hantavirus nephropathy, but there is no certain evidence [3]. In this case, we found Hantavirus-induced nephropathy with characteristic hemorrhagic interstitial nephritis accompanied with an IgA deposition which is probably the result of an immune complex. Whether the mesangial IgA deposition was a coincidental glomerular pathology causing a tendency for the proteinuria to reach a nephrotic level or whether there is a causal relation as a result of an immune response to the virus still remains unsolved. Nephrotic range proteinuria in patients with Hantavirus nephropathy could be due to podocyte effacement [4]; however, we were unable to perform electron microscopy to verify this
Case 2
A 69-year-old male presented with watery diarrhea, fatigue, abdominal pain, and fever. He had no other disease history except for a kidney stone 24 years earlier. Checking the remote laboratory results, his hemoglobin level was found to have been 15 g/dL and his creatinine level was 1.02 mg/dL one year ago. It was revealed that 3 days earlier in another center his serum creatinine level was 1.91 mg/dL, thrombocytes 72 × 109/L, hemoglobin 12.1 g/dL, and LDH 527 U/L However, no peripheral blood smear had been performed. In our center’s follow-up, the creatinine level was found to be 3.03 mg/dL, and it increased rapidly to 5.74 mg/dL within 4 days. Physical examination was unremarkable. Because of an acute kidney injury and prior thrombocytopenia, several laboratory tests were carried out: ADAMTS 13 activity was 52%, haptoglobin 2.26 g/L (0.36–1.95), LDH 627 U/L, direct Coombs test was negative, hemoglobin 14.5 g/dL, no schistocytes in the peripheral blood smear, serological markers for hepatitis B and C were negative, CMV was negative. HIV serology was found to be positive. HIV RNA PCR measured 5,277,174 IU/mL. Initial urine analysis showed only proteinuria. Renal ultrasonography revealed two enlarged kidneys with simple cysts. Because of ongoing renal deterioration, a renal biopsy was performed on the tenth day which revealed HIV-associated collapsing glomerulopathy (HIVAN), diffuse infiltrative lymphocytosis syndrome (DILS), and thrombotic microangiopathy (TMA) (Fig. 2). Prednisolone and eculizumab were initiated in addition to HIV treatment (lamivudine and dolutegravir). After 1 week, his serum creatinine and LDH levels decreased to 2.62 mg/dL and 198 U/L, respectively. After 2 months, serum creatinine level was 1.20 mg/dL. Genetic analysis for atypical HUS was carried out and revealed no mutations. Six months later, after HIV RNA became negative, eculizumab and steroids were stopped.
a In the interstitial region, there were severe mononuclear inflammatory infiltrates and the tubules were diffusely infiltrated by lymphocytes along with severe acute tubular injury characterized by microcystic tubular dilatation, scarring, and interstitial edema (H&E × 150). b Overall, some glomeruli were hypercellular with prominent podocytes and collapsed capillary loops, while others were sclerotic, either segmental or global. There was a glomerulus (arrow) with a collapse, occlusion of capillary loops and overlying podocyte hypertrophy (H&E × 310). c Arrows showing two arterioles occluded by intimal edema with entrapped red blood cells and an arteriolar fibrin thrombus in continuity with the vascular pole of a glomerulus that are the evidence of acute TMA (H&E × 310). In this case, the TMA was caused by complement cascade activation by HIV. d Mononuclear infiltrate was rich in CD3 T cells, which is consistent with tubulointerstitial injury in the setting of HIV (Immunohistochemistry × 290). Immunofluorescence examination of the biopsy revealed no specific staining. The treatment of this patient was led by pathological findings. In addition to the HIV treatment, steroids and eculizumab were initiated for interstitial inflammation and thrombotic microangiopathy, respectively. In our case, there were three distinct pathologic entities which may explain why the patient had such severe acute renal injury. HIVAN, which presents as a collapsing variant of focal and segmental glomerulosclerosis, is the most common kidney disease in HIV-infected patients. DILS is part of the spectrum of HIVAN in the kidney, and it is a hyperimmune reaction against HIV antigens. The main therapy for these renal pathologies is highly active anti-retroviral therapy. Steroids should be used for severe tubular inflammation [5]. Before the modern treatment of HIV, severe forms of TMA were more frequent and its prevalence e is underestimated because of the lack of renal biopsies [6]. There is emerging data, as in our case, about using eculizumab safely in HIV-related aHUS. Treatment duration and relapse rate after drug discontinuation in aHUS are yet to be established in HIV
References
Kupin WL (2017) Viral-associated GN: hepatitis B and other viral infections. Clin J Am Soc Nephrol 12(9):1529–1533
Khaiboullina SF, Morzunov SP, St Jeor SC (2005) Hantaviruses: molecular biology, evolution and pathogenesis. Curr Mol Med 5(8):773–790
Ferluga D, Vizjak A (2008) Hantavirus nephropathy. J Am Soc Nephrol 19(9):1653–1658
Boehlke C, Hartleben B, Huber TB, Hopfer H, Walz G, Neumann-Haefelin E (2014) Hantavirus infection with severe proteinuria and podocyte foot-process effacement. Am J Kidney Dis 64(3):452–456
Saab KR, Elhadad S, Copertino D, Laurence J (2016) Thrombotic microangiopathy in the setting of HIV Infection: a case report and review of the differential diagnosis and therapy. AIDS Patient Care STDS 30(8):359–364
Peraldi MN, Maslo C, Akposso K, Mougenot B, Rondeau E, Sraer JD (1999) Acute renal failure in the course of HIV infection: a single-institution retrospective study of ninety-two patients and sixty renal biopsies. Nephrol Dial Transpl 14(6):1578–1585
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
The authors have complied with Ethical standards for these cases.
Informed consent
Written informed consent was obtained from the patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eren Sadioglu, R., Eyupoglu, S., Kiremitci, S. et al. Two patients, two viruses and multiple sites of injury in the kidney. J Nephrol 34, 263–265 (2021). https://doi.org/10.1007/s40620-020-00838-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40620-020-00838-6