Abstract
Purpose
Skeletal muscle (Skm) plays a key role in regulating energetic metabolism through glucose homeostasis. Several hormones such as Testosterone (T) and Vitamin D (VD) have been shown to affect energy-dependent cell trafficking by determining Insulin (I)-like effects.
Aim
To elucidate possible hormone-related differences on muscular metabolic control, we analyzed and compared the effects of T and elocalcitol (elo), a VD analogue, on the activation of energy-dependent cell trafficking, metabolism-related-signal transduction pathways and transcription of gene downstream targets.
Methods
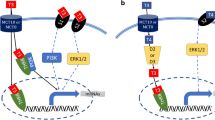
Human fetal skeletal muscle cells (Hfsmc) treated with T or elo were analyzed for GLUT4 localization, phosphorylation/activation status of AKT, ERK1/2, IRS-1 signaling and c-MYC protein expression.
Results
T, similar to elo, induced GLUT4 protein translocation likely in lipid raft microdomains. While both T and elo induced a rapid IRS-1 phosphorylation, the following dynamic in phosphorylation/activation of AKT and ERK1/2 signaling was different. Moreover, T but not elo increased c-MYC protein expression.
Conclusions
All together, our evidence indicates that whether both T and elo are able to affect upstream I-like pathway, they differently determine downstream effects in I-dependent cascade, suggesting diverse physiological roles in mediating I-like response in human skeletal muscle.




Similar content being viewed by others
References
Argilés JM, Campos N, Lopez-Pedrosa JM, Rueda R, Rodriguez-Mañas L (2016) Skeletal muscle regulates metabolism via interorgan crosstalk: roles in health and disease. J Am Med Dir Assoc 1:789–796
Meyer C, Dostou JM, Welle SL, Gerich JE (2002) Role of human liver, kidney, and skeletal muscle in postprandial glucose homeostasis. Am J Physiol Endocrinol Metab 282:E419–E427
Pratesi A, Tarantini F, Di Bari M (2013) Skeletal muscle: an endocrine organ. Clin Cases Miner Bone Metab 10:11–14
Hoffmann C, Weigert C (2017) Skeletal Muscle as an endocrine organ: the role of myokines in exercise adaptations. Cold Spring Harb Perspect Med 7(11):a029793
Sinacore DR, Gulve EA (1993) The role of skeletal muscle in glucose transport, glucose homeostasis, and insulin resistance: implications for physical therapy. Phys Ther 73:878–891
Antinozzi C, Corinaldesi C, Giordano C, Pisano A, Cerbelli B, Migliaccio S, Di Luigi L, Stefanantoni K, Vannelli GB, Minisola S, Valesini G, Riccieri V, Lenzi A, Crescioli C (2017) Potential role for the VDR agonist elocalcitol in metabolic control: evidences in human skeletal muscle cells. J Steroid Biochem Mol Biol 167:169–181
Antinozzi C, Marampon F, Corinaldesi C, Vicini E, Sgrò P, Vannelli GB, Lenzi A, Crescioli C, Di Luigi L (2017) Testosterone insulin-like effects: an in vitro study on the short-term metabolic effects of testosterone in human skeletal muscle cells. J Endocrinol Invest 40:1133–1143
Sato K, Iemitsu M, Aizawa K, Ajisaka R (2008) Testosterone and DHEA activate the glucose metabolism-related signaling pathway in skeletal muscle. Am J Physiol Endocrinol Metab 294:961–968
Inada A, Fujii NL, Inada O, Higaki Y, Furuichi Y, Nabeshima Y (2016) Effects of 17β-Estradiol and androgen on glucose metabolism in skeletal muscle. Endocrinology 157(12):4691–4705
Ceci R, Duranti G, Sgrò P, Sansone M, Guidetti L, Baldari C, Sabatini S, Di Luigi L (2015) Effects of tadalafil administration on plasma markers of exercise-induced muscle damage, IL6 and antioxidant status capacity. Eur J Appl Physiol 115:531–539
Kelly DM, Jones TH (2013) Testosterone: a metabolic hormone in health and disease. J Endocrinol 29:R25–R45
Rao PM, Kelly DM, Jones TH (2013) Testosterone and insulin resistance in the metabolic syndrome and T2DM in men. Nat Rev Endocrinol 9:479–493
Scolletta S, Colletti M, Di Luigi L, Crescioli C (2013) Vitamin D receptor agonists target CXCL10: new therapeutic tools for resolution of inflammation. Mediators Inflamm 2013:876319
Vasile M, Corinaldesi C, Antinozzi C, Crescioli C (2017) Vitamin D in autoimmune rheumatic diseases: a view inside gender differences. Pharmacol Res 117:228–241
Crescioli C (2014) Vitamin D receptor agonists: suitable candidates as novel therapeutic options in autoimmune inflammatory myopathy. Biomed Res Int 2014:949730
Di Luigi L, Romanelli F, Sgrò P, Lenzi A (2012) Andrological aspects of physical exercise and sport medicine. Endocrine 42:278–284
Strange RC, Shipman KE, Sudarshan Ramachandran S (2015) Metabolic syndrome: a review of the role of Vitamin D in mediating susceptibility and outcome. World J Diabetes 10:896–911
Makhsida N, Shah J, Yan G, Fisch H, Shabsigh R (2005) Hypogonadism and metabolic syndrome: implications for testosterone therapy. J Urol 174:827–834
Maktabi M, Chamani M, Asemi Z (2017) The effects of vitamin D supplementation on metabolic status of patients with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Horm Metab Res 49:493–498
Corona G, Giagulli VA, Maseroli E, Vignozzi L, Aversa A, Zitzmann M, Saad F, Mannucci E, Maggi M (2016) Therapy of endocrine disease: testosterone supplementation and body composition: results from a meta-analysis study. Eur J Endocrinol 174(3):R99–R116
Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP (2014) Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ 1:348
Akbari M, Moosazaheh M, Lankarani KB, Tabrizi R, Samimi M, Karamali M, Jamilian M, Kolahdooz F, Asemi Z (2017) The effects of vitamin D supplementation on glucose metabolism and lipid profiles in patients with gestational diabetes: a systematic review and meta-analysis of randomized controlled trials. Horm Metab Res 49(9):647–653
Lips P, Eekhoff M, van Schoor N, Oosterwerff M, de Jongh R, Krul-Poel Y, Simsek S (2017) Vitamin D and type 2 diabetes. J Steroid Biochem Mol Biol 173:280–285
Crescioli C, Ferruzzi P, Caporali A, Scaltriti M, Bettuzzi S, Mancina R, Gelmini S, Serio M, Villari D, Vannelli GB, Colli E, Adorini L, Maggi M (2004) Inhibition of prostate cell growth by BXL-628, a calcitriol analogue selected for a phase II clinical trial in patients with benign prostate hyperplasia. Eur J Endocrinol 150:591–603
Marchiani S, Bonaccorsi L, Ferruzzi P, Crescioli C, Muratori M, Adorini L, Forti G, Maggi M, Baldi E (2006) The vitamin D analogue BXL-628 inhibits growth factor-stimulated proliferation and invasion of DU145 prostate cancer cells. J Cancer Res Clin Oncol 132:408–416
Crescioli C, Sottili M, Bonini P, Cosmi L, Chiarugi P, Romagnani P, Vannelli GB, Colletti M, Isidori AM, Serio M, Lenzi A, Di Luigi L (2012) Inflammatory response in human skeletal muscle cells: CXCL10 as a potential therapeutic target. Eur J Cell Biol 91:139–149
Marampon F, Antinozzi C, Corinaldesi C, Vannelli GB, Sarchielli E, Migliaccio S, Di Luigi L, Lenzi A, Crescioli C (2018) The phosphodiesterase 5 inhibitor tadalafil regulates lipidic homeostasis in human skeletal muscle cell metabolism. Endocrine 59:602–613
Di Luigi L, Sottili M, Antinozzi C, Vannelli GB, Romanelli F, Riccieri V, Valesini G, Lenzi A, Crescioli C (2013) The vitamin D receptor agonist BXL-01-0029 as a potential new pharmacological tool for the treatment of inflammatory myopathies. PLoS One 8:e77745
Crescioli C, Sturli N, Sottili M, Bonini P, Lenzi A, Di Luigi L (2013) Insulin-like effect of the phosphodiesterase type 5 inhibitor tadalafil onto male human skeletal muscle cells. J Endocrinol Invest 36:1020–1026
Bhasin S, Storer TW, Berman N, Callegari C, Clevenger B, Phillips J, Bunnell TJ, Tricker R, Shirazi A, Casaburi RN (1996) The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men. Engl J Med 4(1):1–7
Mannelli M, Ferruzzi P, Luciani P, Crescioli C, Buci L, Corona G, Serio M, Peri A (2003) Cushing’s syndrome in a patient with bilateral macronodular adrenal hyperplasia responding to cisapride: an in vivo and in vitro study. J Clin Endocrinol Metab 88(10):4616–4622
Huang S, Czech MP (2007) The GLUT4 glucose transporter. Cell Metab 5:237–252
Zorzano A, Palacín M, Gumà A (2005) Mechanisms regulating GLUT4 glucose transporter expression and glucose transport in skeletal muscle. Acta Physiol Scand 183:43–58
Ryder JW, Chibalin AV, Zierath JR (2001) Intracellular mechanisms underlying increases in glucose uptake in response to insulin or exercise in skeletal muscle. Acta Physiol Scand 171:249–257
Long YC, Cheng Z, Copps KD, White MF (2011) Insulin receptor substrates Irs1 and Irs2 coordinate skeletal muscle growth and metabolism via the Akt and AMPK pathways. Mol Cell Biol 31:430–441
Valera A, Pujol A, Gregori X, Riu E, Visa J, Bosch F (1995) Evidence from transgenic mice that myc regulates hepatic glycolysis. FASEB J 9:1067–1078
Riu E, Bosch F, Valera A (1996) Prevention of diabetic alterations in transgenic mice overexpressing Myc in the liver. Proc Natl Acad Sci USA 93:2198–2202
DeFronzo RA (1997) Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Diabetes Rev 3:177–269
DeFronzo RA (1988) The triumvirate: β-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes 37:667–687
DeFronzo RA (2004) Pathogenesis of type 2 diabetes mellitus. Med Clin N Am 88:787–835
DeFronzo RA (2009) From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58:773–795
De Fronzo RA, Tripathy D (2009) Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 32:S157–S163
Fornari R, Marocco C, Francomano D, Fittipaldi S, Lubrano C, Bimonte VM, Donini LM, Nicolai E, Aversa A, Lenzi A, Greco EA, Migliaccio S (2018) Insulin growth factor-1 correlates with higher bone mineral density and lower inflammation status in obese adult subjects. Eat Weight Disord 23:375–381
Chiu KC, Chu A, Go VL, Saad MF (2004) Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr 79:820–825
Rao PM, Kelly DM, Jones TH (2013) Testosterone and insulin resistance in the metabolic syndrome and T2DM in men. Nat Rev Endocrinol 9:479–493
Shepherd PR, Kahn BB (1999) Glucose transporters and insulin action—implications for insulin resistance and diabetes mellitus. N Engl J Med 341:248–257
Pessin JE, Thurmond DC, Elmendorf JS, Coker KJ, Okada S (1999) Molecular basis of insulin-stimulated GLUT4 vesicle trafficking. J Biol Chem 274:2593–2596
Ros-Baro A, Lopez-Iglesias C, Peiro S, Bellido D, Palacin M, Zorzano A, Camps M (2001) Lipid rafts are required for GLUT4 internalization in adipose cells. Proc Natl Acad Sci USA 98:12050–12055
Yuan T, Hong S, Yao Y, Liao K (2007) Glut-4 is translocated to both caveolae and non-caveolar lipid rafts, but is partially internalized through caveolae in insulin-stimulated adipocytes. Cell Res 17:772–782
Ijuin T, Takenawa T (2012) Regulation of insulin signaling and glucose transporter 4 (GLUT4) exocytosis by phosphatidylinositol 3,4,5-trisphosphate (PIP3) phosphatase, skeletal muscle, and kidney enriched inositol polyphosphate phosphatase (SKIP). J Biol Chem 28:6991–6999
Khamzina L, Gruppuso PA, Wands JR (2003) Insulin signaling through insulin receptor substrate 1 and 2 in normal liver development. Gastroenterology 25:572–585
Boucher J, Kleinridders A, Kahn CR (2014) Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol 6(1):a009191
Simoncini T, Genazzani AR (2003) Non-genomic actions of sex steroid hormones. Eur J Endocrinol 148:281–292
Simoncini T, Mannella P, Fornari L, Caruso A, Varone G, Genazzani AR (2004) Genomic and non-genomic effects of estrogens on endothelial cells. Steroids 69:537–542
Avruch J (2007) MAP kinase pathways: the first twenty years. Biochim Biophys Acta 1773:1150–1160
Meloche S, Pouysségur J (2007) The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene 14:3227–3239
Girgis CM, Clifton-Bligh RJ, Mokbel N, Cheng K, Endocrinology GJ (2014) Vitamin D signaling regulates proliferation, differentiation, and myotube size in C2C12 skeletal muscle cells. Endocrinology 155:347–357
Fu R, Liu J, Fan J, Li R, Li D, Yin J, Cui S (2012) Novel evidence that testosterone promotes cell proliferation and differentiation via G protein-coupled receptors in the rat L6 skeletal muscle myoblast cell line. J Cell Physiol 227:98–107
Kadi F (2008) Cellular and molecular mechanisms responsible for the action of testosterone on human skeletal muscle. A basis for illegal performance enhancement. Br J Pharmacol 154:522–528
Srikuea R, Esser KA, Pholpramool C (2011) Leukaemia inhibitory factor is expressed in rat gastrocnemius muscle after contusion and increases proliferation of rat L6 myoblasts via c-Myc signaling. Clin Exp Pharmacol Physiol 38:501–509
Nicolini C (2013) Molecular basis of human cancer. Springer, Berlin
Gravina GL, Festuccia C, Popov VM, Di Rocco A, Colapietro A, Sanità P, Monache SD, Musio D, De Felice F, Di Cesare E, Tombolini V, Marampon F (2016) c-Myc sustains transformed phenotype and promotes radioresistance of embryonal rhabdomyosarcoma cell lines. Radiat Res 185:411–422
Sejersen T, Sumegi J, Ringertz NR (1985) Densitydependent arrest of DNA replication is accompanied by decreased levels of c-myc mRNA in myogenic but not in differentiation-defective myoblasts. J Cell Physiol 125:465–470
Sears RC (2004) The life cycle of C-myc: from synthesis to degradation. Cell Cycle 3:1133–1137
Mark A, Gregory MA, Hann SR (2000) c-Myc proteolysis by the ubiquitin-proteasome pahway: stabilization of c-Myc in Burkitt’s lymphoma cells. Mol Cell Biol 20:2423–2435
Sears R, Nuckolls F, Haura E, Taya Y, Tamai K, Nevins JR (2000) Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes Dev 14:2501–2514
Marampon F, Ciccarelli C, Zani BM (2006) Down-regulation of c-Myc following MEK/ERK inhibition halts the expression of malignant phenotype in rhabdomyosarcoma and in non muscle-derived human tumors. Mol Cancer 5:31
Morton RW, Sato K, Gallaugher MPB, Oikawa SY, McNicholas PD, Fujita S, Phillips SM (2018) Muscle androgen receptor content but not systemic hormones is associated with resistance training-induced skeletal muscle hypertrophy in healthy, young men. Front Physiol 9:1373
Larionov AA, Vasyliev DA, Mason JI, Howie AF, Berstein LM, Miller WR (2003) Aromatase in skeletal muscle. J Steroid Biochem Mol Biol 84:485–492
Hevener AL, Zhou Z, Moore TM, Drew BG, Ribas V (2018) The impact of ERα action on muscle metabolism and insulin sensitivity—strong enough for a man, made for a woman. Mol Metab 15:20–34
Geraghty RJ, Capes-Davis A, Davis JM, Downward J, Freshney RI, Knezevic I, Lovell-Badge R, Masters W Jr, Meredith J, Stacey GN, Thraves P, Vias M (2014) Guidelines for the use of cell lines in biomedical research. Br J Cancer 111:1021–1046
Borgogni E, Sarchielli E, Sottili M, Santarlasci V, Cosmi L, Gelmini S, Lombardi A, Cantini G, Perigli G, Luconi M, Vannelli GB, Annunziato F, Adorini L, Serio M, Crescioli C (2008) Elocalcitol inhibits inflammatory responses in human thyroid cells and T cells. Endocrinology 149:3626–3634
Crescioli C, Ferruzzi P, Caporali A, Scaltriti M, Bettuzzi S, Mancina R, Gelmini S, Serio M, Villari D, Vannelli GB, Colli E, Adorini L, Maggi M (2004) Inhibition of prostate cell growth by BXL-628, a calcitriol analogue selected for a phase II clinical trial in patients with benign prostate hyperplasia. Eur J Endocrinol 150:591–603
Sagrinati C, Sottili M, Mazzinghi B, Borgogni E, Adorini L, Serio M, Romagnani P, Crescioli C (2010) Comparison between VDR analogs and current immunosuppressive drugs in relation to CXCL10 secretion by human renal tubular cells. Transpl Int 23:914–923
Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, Chen X, Yarasheski KE, Magliano L, Dzekov C, Dzekov J, Bross R, Phillips J, Sinha-Hikim I, Shen R, Storer TW (2001) Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab 281:E1172–E1181
Vogel RB, Books CA, Ketchum C, Zauner CW, Murray FT (1985) Increase of free and total testosterone during submaximal exercise in normal males. Med Sci Sports Exerc 17:119–123
Devi S, Saxena J, Rastogi D, Goel A, Saha S (2014) Effect of short-term physical exercise on serum total testosterone levels in young adults. Indian J Physiol Pharmacol 58:178–181
Zouhal H (2010) Androgen responses to sprint exercise in young men. Int J Sports Med 31:291–297
Tremblay MS, Copeland JL, Van Helder W (2005) Influence of exercise duration on post-exercise steroid hormone responses in trained males. Eur J Appl Physiol 94:505–513
Kraemer WJ, Ratamess NA (2005) Hormonal responses and adaptations to resistance exercise and training. Sports Med 35:339–361
Tripathi MK, Singh R (2014) Differential suppressive effects of testosterone on immune function in fresh water snake, Natrix piscator: an in vitro study. PLoS 9(8):e104431
Allemand MC, Irving BA, Asmann YW, Klaus KA, Tatpati L, Coddington CC, Nair KS (2009) Effect of testosterone on insulin stimulated IRS1 Ser phosphorylation in primary rat myotubes–a potential model for PCOS-related insulin resistance. PLoS One 4:e4274
Fan SL, Schroeder NJ, Calverley MJ, Burrin JM, Makin HL, Cunningham J (2000) Potent suppression of the parathyroid glands by hydroxylated metabolites of dihydrotachysterol. Nephrol Dial Transplant 15:1943–1949
Brown AJ, Ritter CS, Knutson JC, Strugnell SA (2006) The vitamin D prodrugs 1alpha(OH)D2, 1alpha(OH)D3 and BCI-210 suppress PTH secretion by bovine parathyroid cells. Nephrol Dial Transplant 21:644–650
Schiffer L, Kempegowda P, Arlt W, O’Reilly MW (2017) Mechanisms in endocrinology: the sexually dimorphic role of androgens in human metabolic disease. Eur J Endocrinol 177:R125–R143
Ormazabal P, Romero C, Quest AF, Vega M (2013) Testosterone modulates the expression of molecules linked to insulin action and glucose uptake in endometrial cells. Horm Metab Res 45:640–645
Jankowska A, Metelska P, Słomińska-Frączek M, Socha P (2017) Long-term effects of vitamin D supplementation in vitamin D deficient obese children participating in an integrated weight-loss programme (a double-blind placebo-controlled study)—rationale for the study design. BMC Pediatr 17:97
Cai X, Tian Y, Wu T, Cao CX, Li H, Wang KJ (2014) Metabolic effects of testosterone replacement therapy on hypogonadal men with type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Asian J Androl 16:146–152
Acknowledgements
The Authors wish to thank Dr. Francesca Serena Pignataro, University of Rome Foro Italico and IRCCS San Raffaele Pisana, Department of Internal Medicine, Rome for her assistance in coordination/supervision during manuscript revision and re-writing; Dr. Silvia Giannattasio, University of Rome Foro Italico, for her assistance in reference editing. This research was supported by the grant PRIN (prot. 2010C8ERKX_002) from “University of Rome “Foro Italico”, P. I. Luigi Di Luigi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
No informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Antinozzi, C., Marampon, F., Sgrò, P. et al. Comparative study of testosterone and vitamin D analogue, elocalcitol, on insulin-controlled signal transduction pathway regulation in human skeletal muscle cells. J Endocrinol Invest 42, 897–907 (2019). https://doi.org/10.1007/s40618-018-0998-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40618-018-0998-6