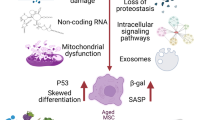
Abstract
Purpose of Review
Mesenchymal stem cells (MSCs) have been extensively studied for therapeutic application in tissue engineering and regenerative medicine. Despite their promise, recent findings suggest that MSC replication during repair process may lead to replicative senescence and stem cell exhaustion. Here, we review the basic mechanisms of MSC senescence, how it leads to degenerative diseases, and potential treatments for such diseases.
Recent Findings
Emerging evidence has shown a link between senescent MSCs and degenerative diseases, especially age-related diseases such as osteoarthritis and idiopathic pulmonary fibrosis. During these disease processes, MSCs undergo cell senescence and mediate Senescence Associated Secretory Phenotypes (SASP) to affect the surrounding microenvironment. Thus, senescent MSCs can accelerate tissue aging by increasing the number of senescent cells and spreading inflammation to neighboring cells.
Summary
Senescent MSCs not only hamper tissue repair through cell senescence associated stem cell exhaustion but also mediate tissue degeneration by initiating and spreading senescence-associated inflammation. It suggests new strategies of MSC-based cell therapy to remove, rejuvenate, or replace (3Rs) the senescent MSCs.


Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. https://doi.org/10.1038/s41536-019-0083-6.
Zhou X, Hong Y, Zhang H, Li X. Mesenchymal stem cell senescence and rejuvenation: current status and challenges. Front Cell Dev Biol. 2020;8(364). https://doi.org/10.3389/fcell.2020.00364.
Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: an update. Cell Transplant. 2016;25(5):829–48. https://doi.org/10.3727/096368915x689622.
Dimmeler S, Leri A. Aging and disease as modifiers of efficacy of cell therapy. Circ Res. 2008;102(11):1319–30. https://doi.org/10.1161/circresaha.108.175943.
Li Y, Wu Q, Wang Y, Li L, Bu H, Bao J. Senescence of mesenchymal stem cells (review). Int J Mol Med. 2017;39(4):775–82. https://doi.org/10.3892/ijmm.2017.2912.
Neri S, Borzì RM. Molecular mechanisms contributing to mesenchymal stromal cell aging. Biomolecules. 2020;10(2). https://doi.org/10.3390/biom10020340.
Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. https://doi.org/10.1016/0014-4827(61)90192-6.
Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, Leboff MS, et al. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7(3):335–43. https://doi.org/10.1111/j.1474-9726.2008.00377.x.
Herbig U, Ferreira M, Condel L, Carey D, Sedivy JM. Cellular senescence in aging primates. Science. 2006;311(5765):1257. https://doi.org/10.1126/science.1122446.
• Franzen J, Zirkel A, Blake J, Rath B, Benes V, Papantonis A, et al. Senescence-associated DNA methylation is stochastically acquired in subpopulations of mesenchymal stem cells. Aging Cell. 2017;16(1):183–91. https://doi.org/10.1111/acel.12544. This work provides evidence of epigenetic modification of DNA associated with aging of MSCs.
Andrzejewska A, Lukomska B, Janowski M. Concise review: Mesenchymal stem cells: from roots to boost. Stem Cells. 2019;37(7):855–64. https://doi.org/10.1002/stem.3016.
• Hu N, Gao Y, Jayasuriya CT, Liu W, Du H, Ding J, et al. Chondrogenic induction of human osteoarthritic cartilage-derived mesenchymal stem cells activates mineralization and hypertrophic and osteogenic gene expression through a mechanomiR. Arthritis Res Ther. 2019;21(1):167. https://doi.org/10.1186/s13075-019-1949-0. This work demonstrates a microRNA dependent mechanism of alteration of differentiation potentials in senescent MSCs.
Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192(4):547–56. https://doi.org/10.1083/jcb.201009094.
Reitinger S, Schimke M, Klepsch S, de Sneeuw S, Yani SL, Gaßner R, et al. Systemic impact molds mesenchymal stromal/stem cell aging. Transfus Apher Sci. 2015;52(3):285–9. https://doi.org/10.1016/j.transci.2015.04.008.
Akyurekli C, Le Y, Richardson RB, Fergusson D, Tay J, Allan DS. A systematic review of preclinical studies on the therapeutic potential of mesenchymal stromal cell-derived microvesicles. Stem Cell Rev Rep. 2015;11(1):150–60. https://doi.org/10.1007/s12015-014-9545-9.
Xie H, Wang Z, Zhang L, Lei Q, Zhao A, Wang H, et al. Development of an angiogenesis-promoting microvesicle-alginate-polycaprolactone composite graft for bone tissue engineering applications. PeerJ. 2016;4:e2040. https://doi.org/10.7717/peerj.2040.
Yuan QL, Zhang YG, Chen Q. Mesenchymal stem cell (MSC)-derived extracellular vesicles: potential therapeutics as MSC trophic mediators in regenerative medicine. Anat Rec (Hoboken). 2020;303(6):1735–42. https://doi.org/10.1002/ar.24186.
Lei Q, Liu T, Gao F, Xie H, Sun L, Zhao A, et al. Microvesicles as potential biomarkers for the identification of senescence in human Mesenchymal stem cells. Theranostics. 2017;7(10):2673–89. https://doi.org/10.7150/thno.18915.
Toussaint O, Medrano EE, von Zglinicki T. Cellular and molecular mechanisms of stress-induced premature senescence (SIPS) of human diploid fibroblasts and melanocytes. Exp Gerontol. 2000;35(8):927–45. https://doi.org/10.1016/s0531-5565(00)00180-7.
Stolzing A, Coleman N, Scutt A. Glucose-induced replicative senescence in mesenchymal stem cells. Rejuvenation Res. 2006;9(1):31–5. https://doi.org/10.1089/rej.2006.9.31.
Severino V, Alessio N, Farina A, Sandomenico A, Cipollaro M, Peluso G, et al. Insulin-like growth factor binding proteins 4 and 7 released by senescent cells promote premature senescence in mesenchymal stem cells. Cell Death Dis. 2013;4(11):e911. https://doi.org/10.1038/cddis.2013.445.
Gharibi B, Farzadi S, Ghuman M, Hughes FJ. Inhibition of Akt/mTOR attenuates age-related changes in Mesenchymal stem cells. Stem Cells. 2014;32(8):2256–66. https://doi.org/10.1002/stem.1709.
Oh J, Lee YD, Wagers AJ. Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat Med. 2014;20(8):870–80. https://doi.org/10.1038/nm.3651.
Goldring MB. Articular cartilage degradation in osteoarthritis. HSS J. 2012;8(1):7–9. https://doi.org/10.1007/s11420-011-9250-z.
Li G, Yin J, Gao J, Cheng TS, Pavlos NJ, Zhang C, et al. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther. 2013;15(6):223. https://doi.org/10.1186/ar4405.
Alsalameh S, Amin R, Gemba T, Lotz M. Identification of mesenchymal progenitor cells in normal and osteoarthritic human articular cartilage. Arthritis Rheum. 2004;50(5):1522–32. https://doi.org/10.1002/art.20269.
Hattori S, Oxford C, Reddi AH. Identification of superficial zone articular chondrocyte stem/progenitor cells. Biochem Biophys Res Commun. 2007;358(1):99–103. https://doi.org/10.1016/j.bbrc.2007.04.142.
Grogan SP, Miyaki S, Asahara H, D'Lima DD, Lotz MK. Mesenchymal progenitor cell markers in human articular cartilage: normal distribution and changes in osteoarthritis. Arthritis Res Ther. 2009;11(3):R85. https://doi.org/10.1186/ar2719.
Fickert S, Fiedler J, Brenner RE. Identification of subpopulations with characteristics of mesenchymal progenitor cells from human osteoarthritic cartilage using triple staining for cell surface markers. Arthritis Res Ther. 2004;6(5):R422–32. https://doi.org/10.1186/ar1210.
Su X, Zuo W, Wu Z, Chen J, Wu N, Ma P, et al. CD146 as a new marker for an increased chondroprogenitor cell sub-population in the later stages of osteoarthritis. J Orthop Res. 2015;33(1):84–91. https://doi.org/10.1002/jor.22731.
Jayasuriya CT, Hu N, Li J, Lemme N, Terek R, Ehrlich MG, et al. Molecular characterization of mesenchymal stem cells in human osteoarthritis cartilage reveals contribution to the OA phenotype. Sci Rep. 2018;8(1):7044. https://doi.org/10.1038/s41598-018-25395-8.
• Malaise O, Tachikart Y, Constantinides M, Mumme M, Ferreira-Lopez R, Noack S, et al. Mesenchymal stem cell senescence alleviates their intrinsic and seno-suppressive paracrine properties contributing to osteoarthritis development. Aging (Albany NY). 2019;11(20):9128–46. https://doi.org/10.18632/aging.102379. This study demonstrates a paracrine mechanism for the senescent MSCs in synovium and bone marrow to induce cartilage degeneration.
Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers. 2017;3:17074. https://doi.org/10.1038/nrdp.2017.74.
Cárdenes N, Álvarez D, Sellarés J, Peng Y, Corey C, Wecht S, et al. Senescence of bone marrow-derived mesenchymal stem cells from patients with idiopathic pulmonary fibrosis. Stem Cell Res Ther. 2018;9(1):257. https://doi.org/10.1186/s13287-018-0970-6.
•• Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, et al. Senolytics improve physical function and increase lifespan in old age. Nat Med. 2018;24(8):1246–56. https://doi.org/10.1038/s41591-018-0092-9. A groundbreaking study to demonstrate the anti-aging effects of senolytics in vivo.
Vicinanza C, Aquila I, Scalise M, Cristiano F, Marino F, Cianflone E, et al. Adult cardiac stem cells are multipotent and robustly myogenic: c-kit expression is necessary but not sufficient for their identification. Cell Death Differ. 2017;24(12):2101–16. https://doi.org/10.1038/cdd.2017.130.
Castaldi A, Dodia RM, Orogo AM, Zambrano CM, Najor RH, Gustafsson ÅB, et al. Decline in cellular function of aged mouse c-kit(+) cardiac progenitor cells. J Physiol. 2017;595(19):6249–62. https://doi.org/10.1113/jp274775.
Lewis-McDougall FC, Ruchaya PJ, Domenjo-Vila E, Shin Teoh T, Prata L, Cottle BJ, et al. Aged-senescent cells contribute to impaired heart regeneration. Aging Cell. 2019;18(3):e12931. https://doi.org/10.1111/acel.12931.
•• Nicaise AM, Wagstaff LJ, Willis CM, Paisie C, Chandok H, Robson P, et al. Cellular senescence in progenitor cells contributes to diminished remyelination potential in progressive multiple sclerosis. Proc Natl Acad Sci U S A. 2019;116(18):9030–9. https://doi.org/10.1073/pnas.1818348116. A nice study to demonstrate the contribution of senescent neural stem cells to multiple sclerosis.
• Hladik D, Höfig I, Oestreicher U, Beckers J, Matjanovski M, Bao X, et al. Long-term culture of mesenchymal stem cells impairs ATM-dependent recognition of DNA breaks and increases genetic instability. Stem Cell Res Ther. 2019;10(1):218. https://doi.org/10.1186/s13287-019-1334-6. This work demonstrates molecular mechanisms of cell senescence in long term culture of MSCs.
Burhans WC, Weinberger M. DNA replication stress, genome instability and aging. Nucleic Acids Res. 2007;35(22):7545–56. https://doi.org/10.1093/nar/gkm1059.
Chen X, Wang L, Hou J, Li J, Chen L, Xia J, et al. Study on the dynamic biological characteristics of human bone marrow Mesenchymal stem cell senescence. Stem Cells Int. 2019;2019:9271595–9. https://doi.org/10.1155/2019/9271595.
Jeong SG, Cho GW. Endogenous ROS levels are increased in replicative senescence in human bone marrow mesenchymal stromal cells. Biochem Biophys Res Commun. 2015;460(4):971–6. https://doi.org/10.1016/j.bbrc.2015.03.136.
Lai TP, Wright WE, Shay JW. Comparison of telomere length measurement methods. Philos Trans R Soc Lond Ser B Biol Sci. 2018;373(1741):20160451. https://doi.org/10.1098/rstb.2016.0451.
Nadeau S, Cheng A, Colmegna I, Rodier F. Quantifying senescence-associated phenotypes in primary multipotent Mesenchymal stromal cell cultures. Methods Mol Biol. 2019;2045:93–105. https://doi.org/10.1007/7651_2019_217.
Herranz N, Gil J. Mechanisms and functions of cellular senescence. J Clin Invest. 2018;128(4):1238–46. https://doi.org/10.1172/jci95148.
Ghanta S, Tsoyi K, Liu X, Nakahira K, Ith B, Coronata AA, et al. Mesenchymal stromal cells deficient in autophagy proteins are susceptible to oxidative injury and mitochondrial dysfunction. Am J Respir Cell Mol Biol. 2017;56(3):300–9. https://doi.org/10.1165/rcmb.2016-0061OC.
Revuelta M, Matheu A. Autophagy in stem cell aging. Aging Cell. 2017;16(5):912–5. https://doi.org/10.1111/acel.12655.
Eckhart L, Tschachler E, Gruber F. Autophagic control of skin aging. Front Cell Dev Biol. 2019;7:143. https://doi.org/10.3389/fcell.2019.00143.
Mortensen M, Watson AS, Simon AK. Lack of autophagy in the hematopoietic system leads to loss of hematopoietic stem cell function and dysregulated myeloid proliferation. Autophagy. 2011;7(9):1069–70. https://doi.org/10.4161/auto.7.9.15886.
Huang X, Zhang H, Liang X, Hong Y, Mao M, Han Q, et al. Adipose-derived Mesenchymal stem cells isolated from patients with abdominal aortic aneurysm exhibit senescence phenomena. Oxidative Med Cell Longev. 2019;2019:1305049–12. https://doi.org/10.1155/2019/1305049.
Kirkland JL, Tchkonia T, Zhu Y, Niedernhofer LJ, Robbins PD. The clinical potential of Senolytic drugs. J Am Geriatr Soc. 2017;65(10):2297–301. https://doi.org/10.1111/jgs.14969.
Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, et al. The Achilles' heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14(4):644–58. https://doi.org/10.1111/acel.12344.
Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, et al. Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors. Aging Cell. 2016;15(3):428–35. https://doi.org/10.1111/acel.12445.
Zhu Y, Doornebal EJ, Pirtskhalava T, Giorgadze N, Wentworth M, Fuhrmann-Stroissnigg H, et al. New agents that target senescent cells: the flavone, fisetin, and the BCL-X(L) inhibitors, A1331852 and A1155463. Aging (Albany NY). 2017;9(3):955–63. https://doi.org/10.18632/aging.101202.
•• Justice JN, Nambiar AM, Tchkonia T, LeBrasseur NK, Pascual R, Hashmi SK, et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–63. https://doi.org/10.1016/j.ebiom.2018.12.052. One of the first clinical trials of senolytic treatment of a human aging disease.
McHugh D, Gil J. Senescence and aging: causes, consequences, and therapeutic avenues. J Cell Biol. 2018;217(1):65–77. https://doi.org/10.1083/jcb.201708092.
Wilson WH, O'Connor OA, Czuczman MS, LaCasce AS, Gerecitano JF, Leonard JP, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11(12):1149–59. https://doi.org/10.1016/s1470-2045(10)70261-8.
Demaria M, O'Leary MN, Chang J, Shao L, Liu S, Alimirah F, et al. Cellular senescence promotes adverse effects of chemotherapy and Cancer relapse. Cancer Discov. 2017;7(2):165–76. https://doi.org/10.1158/2159-8290.Cd-16-0241.
Mosteiro L, Pantoja C, Alcazar N, Marión RM, Chondronasiou D, Rovira M, et al. Tissue damage and senescence provide critical signals for cellular reprogramming in vivo. Science. 2016;354(6315):aaf4445. https://doi.org/10.1126/science.aaf4445.
Ritschka B, Storer M, Mas A, Heinzmann F, Ortells MC, Morton JP, et al. The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev. 2017;31(2):172–83. https://doi.org/10.1101/gad.290635.116.
Yang M, Teng S, Ma C, Yu Y, Wang P, Yi C. Ascorbic acid inhibits senescence in mesenchymal stem cells through ROS and AKT/mTOR signaling. Cytotechnology. 2018;70(5):1301–13. https://doi.org/10.1007/s10616-018-0220-x.
Park SY, Jeong AJ, Kim GY, Jo A, Lee JE, Leem SH, et al. Lactoferrin protects human Mesenchymal stem cells from oxidative stress-induced senescence and apoptosis. J Microbiol Biotechnol. 2017;27(10):1877–84. https://doi.org/10.4014/jmb.1707.07040.
Lin TM, Tsai JL, Lin SD, Lai CS, Chang CC. Accelerated growth and prolonged lifespan of adipose tissue-derived human mesenchymal stem cells in a medium using reduced calcium and antioxidants. Stem Cells Dev. 2005;14(1):92–102. https://doi.org/10.1089/scd.2005.14.92.
Lee JH, Jung HK, Han YS, Yoon YM, Yun CW, Sun HY, et al. Antioxidant effects of Cirsium setidens extract on oxidative stress in human mesenchymal stem cells. Mol Med Rep. 2016;14(4):3777–84. https://doi.org/10.3892/mmr.2016.5706.
Lee JH, Yoon YM, Song KH, Noh H, Lee SH. Melatonin suppresses senescence-derived mitochondrial dysfunction in mesenchymal stem cells via the HSPA1L-mitophagy pathway. Aging Cell. 2020;19(3):e13111. https://doi.org/10.1111/acel.13111.
Kornienko JS, Smirnova IS, Pugovkina NA, Ivanova JS, Shilina MA, Grinchuk TM, et al. High doses of synthetic antioxidants induce premature senescence in cultivated mesenchymal stem cells. Sci Rep. 2019;9(1):1296. https://doi.org/10.1038/s41598-018-37972-y.
Dai Z, Jin Y, Zheng J, Liu K, Zhao J, Zhang S, et al. MiR-217 promotes cell proliferation and osteogenic differentiation of BMSCs by targeting DKK1 in steroid-associated osteonecrosis. Biomed Pharmacother. 2019;109:1112–9. https://doi.org/10.1016/j.biopha.2018.10.166.
Li H, Fan J, Fan L, Li T, Yang Y, Xu H, et al. MiRNA-10b reciprocally stimulates osteogenesis and inhibits adipogenesis partly through the TGF-β/SMAD2 signaling pathway. Aging Dis. 2018;9(6):1058–73. https://doi.org/10.14336/ad.2018.0214.
Fan J, An X, Yang Y, Xu H, Fan L, Deng L, et al. MiR-1292 targets FZD4 to regulate senescence and osteogenic differentiation of stem cells in TE/SJ/mesenchymal tissue system via the Wnt/β-catenin pathway. Aging Dis. 2018;9(6):1103–21. https://doi.org/10.14336/ad.2018.1110.
Galkin F, Zhang B, Dmitriev SE, Gladyshev VN. Reversibility of irreversible aging. Ageing Res Rev. 2019;49:104–14. https://doi.org/10.1016/j.arr.2018.11.008.
Hynes K, Menicanin D, Han J, Marino V, Mrozik K, Gronthos S, et al. Mesenchymal stem cells from iPS cells facilitate periodontal regeneration. J Dent Res. 2013;92(9):833–9. https://doi.org/10.1177/0022034513498258.
Spitzhorn LS, Megges M, Wruck W, Rahman MS, Otte J, Degistirici Ö, et al. Human iPSC-derived MSCs (iMSCs) from aged individuals acquire a rejuvenation signature. Stem Cell Res Ther. 2019;10(1):100. https://doi.org/10.1186/s13287-019-1209-x.
Fernandez-Rebollo E, Franzen J, Goetzke R, Hollmann J, Ostrowska A, Oliverio M, et al. Senescence-associated Metabolomic phenotype in primary and iPSC-derived Mesenchymal stromal cells. Stem Cell Reports. 2020;14(2):201–9. https://doi.org/10.1016/j.stemcr.2019.12.012.
Kornicka K, Marycz K, Marędziak M, Tomaszewski KA, Nicpoń J. The effects of the DNA methyltranfserases inhibitor 5-Azacitidine on ageing, oxidative stress and DNA methylation of adipose derived stem cells. J Cell Mol Med. 2017;21(2):387–401. https://doi.org/10.1111/jcmm.12972.
Assis RIF, Wiench M, Silvério KG, da Silva RA, Feltran GDS, Sallum EA, et al. RG108 increases NANOG and OCT4 in bone marrow-derived mesenchymal cells through global changes in DNA modifications and epigenetic activation. PLoS One. 2018;13(12):e0207873. https://doi.org/10.1371/journal.pone.0207873.
Gao B, Lin X, Jing H, Fan J, Ji C, Jie Q, et al. Local delivery of tetramethylpyrazine eliminates the senescent phenotype of bone marrow mesenchymal stromal cells and creates an anti-inflammatory and angiogenic environment in aging mice. Aging Cell. 2018;17(3):e12741. https://doi.org/10.1111/acel.12741.
Ito T, Teo YV, Evans SA, Neretti N, Sedivy JM. Regulation of cellular senescence by Polycomb chromatin modifiers through distinct DNA damage- and histone methylation-dependent pathways. Cell Rep. 2018;22(13):3480–92. https://doi.org/10.1016/j.celrep.2018.03.002.
Acknowledgments
This work was supported in part by NIH R61 AR076807 and P30 GM122732 (to Q.C.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Yajun Liu declares no potential conflict of interest. Qian Chen holds patents relevant to human cartilage derived mesenchymal stem cells and is a co-founder of NanoDe Therapeutics Inc.
Human and Animal Rights and Informed Consent
This article contains no new studies with human and animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Skeletal Degeneration and Regeneration
Rights and permissions
About this article
Cite this article
Liu, Y., Chen, Q. Senescent Mesenchymal Stem Cells: Disease Mechanism and Treatment Strategy. Curr Mol Bio Rep 6, 173–182 (2020). https://doi.org/10.1007/s40610-020-00141-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40610-020-00141-0