Abstract
Purpose of Review
To describe the current knowledge on the cross-talk between connexins and microRNAs (miRs) in bone cells.
Recent Findings
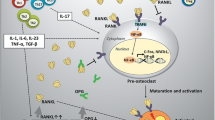
Connexins play a crucial role on bone development and maintenance, and disruptions in their abundance or localization can affect how bone perceives and responds to mechanical, hormonal, and pharmacological stimuli. Connexin expression can be modified by miRs, which modulate connexin mRNA and protein levels. Recently, different manners by which miRs and connexins can interact in the bone have been identified, including mechanisms that mediate miR exchange between cells in direct contact through gap junctions or between distant cells via extracellular vesicles (EVs).
Summary
We bring to light the relationship between miRs and connexins in bone tissue, with special focus on regulatory effects of miRs and connexins on gene expression, as well as the mechanisms that mediate miR exchange between cells in direct contact through gap junctions or between distant cells via EVs.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Plotkin LI, Stains JP. Connexins and pannexins in the skeleton: gap junctions, hemichannels and more. Cell Mol Life Sci. 2015;72:2853–67.
Batra N, Kar R, Jiang JX. Gap junctions and hemichannels in signal transmission, function and development of bone. Biochim Biophys Acta. 2012;1818:1909–18.
Saez JC, Martinez AD, Branes MC, et al. Regulation of gap junctions by protein phosphorylation. Braz J Med Biol Res. 1998;31:593–600.
Valiunas V, Polosina YY, Miller H, et al. Connexin-specific cell to cell transfer of short interfering RNA by gap junctions. J Physiol. 2005;568:459–68.
Lemcke H, Steinhoff G, David R. Gap junctional shuttling of miRNA—a novel pathway of intercellular gene regulation and its prospects in clinical application. Cell Signal. 2015;27:2506–14.
Abels ER, Breakefield XO. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol. 2016;36:301–12.
Xie Y, Chen Y, Zhang L, et al. The roles of bone-derived exosomes and exosomal microRNAs in regulating bone remodelling. J Cell Mol Med. 2016:1–9.
Evans WH. Cell communication across gap junctions: a historical perspective and current developments. Biochem Soc Trans. 2015;43:450–9.
Oshima A. Structure and closure of connexin gap junction channels. FEBS Lett. 2014;588:1230–7.
Martin PE, Evans WH. Incorporation of connexins into plasma membranes and gap junctions. Cardiovasc Res. 2004;62:378–87.
Plotkin LI, Bellido T. Beyond gap junctions: connexin43 and bone cell signaling. Bone. 2013;52:157–66.
Plotkin LI. Connexin 43 hemichannels and intracellular signaling in bone cells. Front Physiol. 2014;5:131.
Paznekas WA, Boyadjiev SA, Shapiro RE, et al. Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am J Hum Genet. 2003;72:408–18.
Koval M, Harley JE, Hick E, et al. Connexin46 is retained as monomers in a trans-Golgi compartment of osteoblastic cells. J Cell Biol. 1997;137:847–57.
Minkoff R, Rundus VR, Parker SB, et al. Gap junction proteins exhibit early and specific expression during intramembranous bone formation in the developing chick mandible. Anat Embryol (Berl). 1994;190:231–41.
Pizard A, Burgon PG, Paul DL, et al. Connexin 40, a target of transcription factor Tbx5, patterns wrist, digits, and sternum. Mol Cell Biol. 2005;25:5073–83.
Pacheco-Costa R, Hassan I, Reginato RD, et al. High bone mass in mice lacking Cx37 due to defective osteoclast differentiation. J Biol Chem. 2014;289:8508–20.
Pacheco-Costa R, Kadakia JR, Atkinson EG, et al. Connexin37 deficiency alters organic bone matrix, cortical bone geometry, and increases Wnt/beta-catenin signaling. Bone. 2017;97:105–13.
Lecanda F, Warlow PM, Sheikh S, et al. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J Cell Biol. 2000;151:931–44.
Plotkin LI, Laird DW, Amedee J. Role of connexins and pannexins during ontogeny, regeneration, and pathologies of bone. BMC Cell Biol. 2016;17:29–38.
Sternlieb M, Paul E, Donahue HJ, et al. Ablation of connexin 43 in osteoclasts leads to decreased in vivo osteoclastogenesis. J Bone Miner Res. 2012;27:S53.
Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–24.
Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–54.
Ambros V, Bartel B, Bartel DP, et al. A uniform system for microRNA annotation. RNA. 2003;9:277–9.
Tetreault N, De G V. MiRNAs: their discovery, biogenesis and mechanism of action. Clin Biochem. 2013;46:842–5.
Garzon R, Calin GA, Croce CM. MicroRNAs in cancer. Annu Rev Med. 2009;60:167–79.
Sun M, Zhou X, Chen L, et al. The regulatory roles of microRNAs in bone remodeling and perspectives as biomarkers in osteoporosis. Biomed Res Int. 2016;2016:1652417.
Ell B, Kang Y. MicroRNAs as regulators of bone homeostasis and bone metastasis. Bonekey Rep. 2014;3:549.
Papaioannou G, Mirzamohammadi F, Kobayashi T. MicroRNAs involved in bone formation. Cell Mol Life Sci. 2014;71:4747–61.
van der Eerden BC. MicroRNAs in the skeleton: cell-restricted or potent intercellular communicators? Arch Biochem Biophys. 2014;561:46–55.
Turchinovich A, Tonevitsky AG, Burwinkel B. Extracellular miRNA: a collision of two paradigms. Trends Biochem Sci. 2016;41:883–92.
Zhang J, Li S, Li L, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics. 2015;13:17–24.
Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles. 2014. 3
Lo CA, Stahl PD, Raposo G. Extracellular vesicles shuffling intercellular messages: for good or for bad. Curr Opin Cell Biol. 2015;35:69–77.
Schwab A, Meyering SS, Lepene B, et al. Extracellular vesicles from infected cells: potential for direct pathogenesis. Front Microbiol. 2015;6:1132.
Buzas EI, Gyorgy B, Nagy G, et al. Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol. 2014;10:356–64.
Ciardiello C, Cavallini L, Spinelli C, et al. Focus on extracellular vesicles: new frontiers of cell-to-cell communication in cancer. Int J Mol Sci. 2016;17
• Soares AR, Martins-Marques T, Ribeiro-Rodrigues T, et al. Gap junctional protein Cx43 is involved in the communication between extracellular vesicles and mammalian cells. Sci Rep. 2015;5:13243. This article shows the presence of Cx43 hemichannels in exosomes and its important participation on the delivery of nucleic acid to recipient cells and their trasnference between exosomes.
Varela-Eirin M, Varela-Vazquez A, Mateos MR. et al. Recruitment of RNA molecules by connexin RNA-binding motifs: implication in RNA and DNA transport through microvesicles and exosomes. Biochim. Biophys. Acta 2017
Inose H, Ochi H, Kimura A, et al. A microRNA regulatory mechanism of osteoblast differentiation. Proc Natl Acad Sci U S A. 2009;106:20794–9.
Liu G, Luo G, Bo Z, et al. Impaired osteogenic differentiation associated with connexin43/microRNA-206 in steroid-induced avascular necrosis of the femoral head. Exp Mol Pathol. 2016;101:89–99.
• Gindin Y, Jiang Y, Francis P, et al. MiR-23a impairs bone differentiation in osteosarcoma via down-regulation of GJA1. Front Genet. 2015;6:233. This article evidence the Cx43 regulation by miR in a osteoblastic cell lineage. This article shows Cx43 regulation by miRs in a cell of osteoblastic lineage.
Ozeki N, Hase N, Hiyama T et al. MicroRNA-211 and autophagy-related gene 14 signaling regulate osteoblast-like cell differentiation of human induced pluripotent stem cells. Exp. Cell Res. 2017
Bejarano E, Yuste A, Patel B. et al. Connexins modulate autophagosome biogenesis. Nat. Cell Biol. 2014
•• Davis HM, Pacheco-Costa R, Atkinson EG, et al. Disruption of the Cx43/miR21 pathway leads to osteocyte apoptosis and increased osteoclastogenesis with aging. Aging Cell. 2017; doi:10.1111/acel.12586. This article shows the requirement of Cx43 and miR21 to maintain osteocyte survival and identified RANKL and HMGB1 as two molecules involved with elevated osteoclastogenesis. In addition, provides the first description of miR regulation via connexin in osteocytic cells.
Zong L, Zhu Y, Liang R, et al. Gap junction mediated miRNA intercellular transfer and gene regulation: a novel mechanism for intercellular genetic communication. Sci. Rep. 2016;6:19884.
Lim PK, Bliss SA, Patel SA, et al. Gap junction-mediated import of microRNA from bone marrow stromal cells can elicit cell cycle quiescence in breast cancer cells. Cancer Res. 2011;71:1550–60.
Vojtech L, Woo S, Hughes S, et al. Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions. Nucleic Acids Res. 2014;42:7290–304.
Deng L, Wang Y, Peng Y, et al. Osteoblast-derived microvesicles: a novel mechanism for communication between osteoblasts and osteoclasts. Bone. 2015;79:37–42.
Cui Y, Luan J, Li H, et al. Exosomes derived from mineralizing osteoblasts promote ST2 cell osteogenic differentiation by alteration of microRNA expression. FEBS Lett. 2016;590:185–92.
Lauvrak SU, Munthe E, Kresse SH, et al. Functional characterisation of osteosarcoma cell lines and identification of mRNAs and miRNAs associated with aggressive cancer phenotypes. Br J Cancer. 2013;109:2228–36.
Hassan MQ, Maeda Y, Taipaleenmaki H, et al. MiR-218 directs a Wnt signaling circuit to promote differentiation of osteoblasts and osteomimicry of metastatic cancer cells. J Biol Chem. 2012;287:42084–92.
Bhushan R, Grunhagen J, Becker J, et al. MiR-181a promotes osteoblastic differentiation through repression of TGF-beta signaling molecules. Int. J. Biochem. Cell Biol. 2013;45:696–705.
Kim YJ, Bae SW, Yu SS, et al. MiR-196a regulates proliferation and osteogenic differentiation in mesenchymal stem cells derived from human adipose tissue. J Bone Miner Res. 2009;24:816–25.
Qin Y, Wang L, Gao Z, et al. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci Rep. 2016;6:21961.
Egea V, Zahler S, Rieth N, et al. Tissue inhibitor of metalloproteinase-1 (TIMP-1) regulates mesenchymal stem cells through let-7f microRNA and Wnt/beta-catenin signaling. Proc Natl Acad Sci U S A. 2012;109:E309–16.
Wei J, Li H, Wang S, et al. Let-7 enhances osteogenesis and bone formation while repressing adipogenesis of human stromal/mesenchymal stem cells by regulating HMGA2. Stem Cells Dev. 2014;23:1452–63.
Schaap-Oziemlak AM, Raymakers RA, Bergevoet SM, et al. MicroRNA hsa-miR-135b regulates mineralization in osteogenic differentiation of human unrestricted somatic stem cells. Stem Cells Dev. 2010;19:877–85.
Xu S, Cecilia SG, De VK, et al. Upregulation of miR-135b is involved in the impaired osteogenic differentiation of mesenchymal stem cells derived from multiple myeloma patients. PLoS One. 2013;8:e79752.
Huang J, Zhao L, Xing L, et al. MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cells. 2010;28:357–64.
Xu JF, Yang GH, Pan XH, et al. Altered microRNA expression profile in exosomes during osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. PLoS One. 2014;9:e114627.
Zhang J, Tu Q, Bonewald LF, et al. Effects of miR-335-5p in modulating osteogenic differentiation by specifically downregulating Wnt antagonist DKK1. J Bone Miner Res. 2011;26:1953–63.
You L, Gu W, Chen L, et al. MiR-378 overexpression attenuates high glucose-suppressed osteogenic differentiation through targeting CASP3 and activating PI3K/Akt signaling pathway. Int J Clin Exp Pathol. 2014;7:7249–61.
Zhang Y, Xie RL, Croce CM, et al. A program of microRNAs controls osteogenic lineage progression by targeting transcription factor Runx2. Proc Natl Acad Sci U S A. 2011;108:9863–8.
Li Z, Hassan MQ, Volinia S, et al. A microRNA signature for a BMP2-induced osteoblast lineage commitment program. Proc Natl Acad Sci U S A. 2008;105:13906–11.
Hwang S, Park SK, Lee HY, et al. MiR-140-5p suppresses BMP2-mediated osteogenesis in undifferentiated human mesenchymal stem cells. FEBS Lett. 2014;588:2957–63.
Cheng P, Chen C, He HB, et al. MiR-148a regulates osteoclastogenesis by targeting V-maf musculoaponeurotic fibrosarcoma oncogene homolog B. J Bone Miner Res. 2013;28:1180–90.
Chen C, Cheng P, Xie H, et al. MiR-503 regulates osteoclastogenesis via targeting RANK. J Bone Miner Res. 2014;29:338–47.
Zhao C, Sun W, Zhang P, et al. MiR-214 promotes osteoclastogenesis by targeting Pten/PI3k/Akt pathway. RNA Biol. 2015;12:343–53.
•• Li D, Liu J, Guo B, et al. Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat Commun. 2016;7:10872. This article shows the osteoclast-derived exosomes released from ovariectomized mice and elderly fractured women contain a miR able to target osteoblasts and then inhibit bone formation.
Kim YH, Goh TS, Lee CS. et al. Prognostic value of microRNAs in osteosarcoma: a meta-analysis. Oncotarget. 2017
Peng S, Gao D, Gao C. et al. MicroRNAs regulate signaling pathways in osteogenic differentiation of mesenchymal stem cells (Review). Mol. Med. Rep. 2016
Burke J, Kolhe R, Hunter M, et al. Stem cell-derived exosomes: a potential alternative therapeutic agent in orthopaedics. Stem Cells Int. 2016;2016:5802529.
Burke J, Hunter M, Kolhe R, et al. Therapeutic potential of mesenchymal stem cell based therapy for osteoarthritis. Clin Transl Med. 2016;5:27.
Sasso L, Hosamuddin H, Emanueli C. Extracellular vesicles at the cross-line between basic science and clinical needs. Microcirculation. 2017. 24
Valiunas V, Doronin S, Valiuniene L, et al. Human mesenchymal stem cells make cardiac connexins and form functional gap junctions. J Physiol. 2004;555:617–26.
Hahn JY, Cho HJ, Kang HJ, et al. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. J Am Coll Cardiol. 2008;51:933–43.
Wang KX, Xu LL, Rui YF, et al. The effects of secretion factors from umbilical cord derived mesenchymal stem cells on osteogenic differentiation of mesenchymal stem cells. PLoS One. 2015;10:e0120593.
Qi X, Zhang J, Yuan H, et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int J Biol Sci. 2016;12:836–49.
Huang-Doran I, Zhang CY, Vidal-Puig A. Extracellular vesicles: novel mediators of cell communication in metabolic disease. Trends Endocrinol Metab. 2017;28:3–18.
Heldring N, Mager I, Wood MJ, et al. Therapeutic potential of multipotent mesenchymal stromal cells and their extracellular vesicles. Hum Gene Ther. 2015;26:506–17.
Acknowledgements
This research was supported by the National Institutes of Health (R01-AR067210 and R01-AR053643 to LIP). HMD is supported by an NIH T32-AR065971 grant. RPC received a scholarship from the Coordination of Improvement of Higher Level Personnel (CAPES), Brazil (PDE# 232636/2014-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Lilian I. Plotkin, Rafael Pacheco-Costa, and Hannah M. Davis declare that they have no potential conflicts of interest.
Human and Animal Rights and Informed Consent
The protocols involving mice were approved by the Institutional Animal Care and Use Committee of the Indiana University School of Medicine. This article does not contain studies with human subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Molecular Biology of Skeletal Development
Rights and permissions
About this article
Cite this article
Plotkin, L.I., Pacheco-Costa, R. & Davis, H.M. MicroRNAs and Connexins in Bone: Interaction and Mechanisms of Delivery. Curr Mol Bio Rep 3, 63–70 (2017). https://doi.org/10.1007/s40610-017-0058-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40610-017-0058-6