Abstract
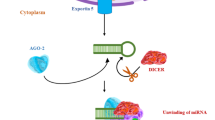
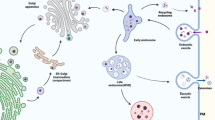
The prevention of neurodevelopmental disorders (NDD) of prenatal origin suffers from the lack of objective tools for early detection of susceptible individuals and the long time lag, usually in years, between the neurotoxic exposure and the diagnosis of mental dysfunction. Human data on the effects of alcohol, lead, and mercury and experimental data from animals on developmental neurotoxins and their long-term behavioral effects have achieved a critical mass, leading to the concept of the Developmental Origin of Health and Disease (DOHaD). However, there is currently no way to evaluate the degree of brain damage early after birth. We propose that extracellular vesicles (EVs) and particularly exosomes, released by brain cells into the fetal blood, may offer us a non-invasive means of assessing brain damage by neurotoxins. We are inspired by the strategy applied by Alan Turing (a cryptanalyst working for the British government), who created a first computer to decrypt German intelligence communications during World War II. Given the growing evidence that microRNAs (miRNAs), which are among the molecules carried by EVs, are involved in cell-cell communication, we propose that decrypting messages from EVs can allow us to detect damage thus offering an opportunity to cure, reverse, or prevent the development of NDD. This review summarizes recent findings on miRNAs associated with selected environmental toxicants known to be involved in the pathophysiology of NDD.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of outstanding importance
Ratnaike R. Acute and chronic arsenic toxicity. Postgrad Med J. 2003;79(933):391–6.
Sanders AP, Claus Henn B, Wright RO. Perinatal and childhood exposure to cadmium, manganese, and metal mixtures and effects on cognition and behavior: a review of recent literature. Curr Environ Health Rep. 2015;2(3):284–94.
Natarajan SK, Pachunka JM, Mott JL. Role of microRNAs in alcohol-induced multi-organ injury. Biomolecules. 2015;5(4):3309–38.
Froehlich TE, Anixt JS, Loe IM, et al. Update on environmental risk factors for attention-deficit/hyperactivity disorder. Curr Psych Rep. 2011;13(5):333–44.
Doyle LR, Mattson SN. Neurobehavioral disorder associated with prenatal alcohol exposure (ND-PAE): review of evidence and guidelines for assessment. Curr Dev Disord Rep. 2015;2(3):175–86.
Grandjean P, Weihe P, Debes F, et al. Neurotoxicity from prenatal and postnatal exposure to methylmercury. Neurotoxicol Teratol. 2014;43:39–44.
Forns J, Fort M, Casas M, Cáceres A, Guxens M, Gascon M, et al. Exposure to metals during pregnancy and neuropsychological development at the age of 4 years. Neurotoxicology. 2014;40:16–22.
Vrijens K, Bollati V, Nawrot TS. MicroRNAs as potential signatures of environmental exposure or effect: a systematic review. Environ Health Perspect. 2015;123(5):399–411. This reviewed provides recent state of knowledge regarding the effects of environmental exposures on miRNAs expression. This suggested that miRNAs could be use as new biomarkers to detect environmental exposures-induced physiological changes.
Hou L, Wang D, Baccarelli A. Environmental chemicals and microRNAs. Mutat Res. 2011;714(1–2):105–12.
Zhang J, Li S, Li L, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinforma. 2015;13(1):17–24.
Pritchard CC, Cheng HH, Tewari M. MicroRNA profiling: approaches and considerations. Nat Rev. 2012;13(5):358–69.
Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15(8):509–24.
Stefani G, Slack FJ. Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol. 2008;9(3):219–30.
Simpson LJ, Ansel KM. MicroRNA regulation of lymphocyte tolerance and autoimmunity. J Clin Invest. 2015;125(6):2242–9.
Heegaard NHH, Carlsen AL, Skovgaard K, Heegaard PMH. Circulating extracellular microRNA in systemic autoimmunity. EXS. 2015;106:171–95.
Lee H-M, Nguyen DT, Lu L-F. Progress and challenge of microRNA research in immunity. Front Genet. 2014;5:178.
Zhu S, Pan W, Qian Y. MicroRNA in immunity and autoimmunity. J Mol Med Berl Ger. 2013;91(9):1039–50.
Arunachalam G, Upadhyay R, Ding H, Triggle CR. MicroRNA signature and cardiovascular dysfunction. J Cardiovasc Pharmacol. 2015;65(5):419–29.
Pfeifer P, Werner N, Jansen F. Role and function of microRNAs in extracellular vesicles in cardiovascular biology. BioMed Res Int. 2015;2015:161393.
Elia L, Condorelli G. RNA (epi)genetics in cardiovascular diseases. J Mol Cell Cardiol. 2015;89(Pt A):11–6.
Kadamkode V, Banerjee G. Micro RNA: an epigenetic regulator of type 2 diabetes. MicroRNA Shāriqah United Arab Emir. 2014;3(2):86–97.
Park S-Y, Jeong H-J, Yang W-M, et al. Implications of microRNAs in the pathogenesis of diabetes. Arch Pharm Res. 2013;36(2):154–66.
Cuellar TL, McManus MT. MicroRNAs and endocrine biology. J Endocrinol. 2005;187(3):327–32.
Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer. 2015;15(6):321–33.
Reddy KB. MicroRNA (miRNA) in cancer. Cancer Cell Int. 2015;15:38.
Chung AC, Yu X, Lan HY. MicroRNA and nephropathy: emerging concepts. Int J Nephrol Renov Dis. 2013;6:169–79.
Kenny PJ. Epigenetics, microRNA, and addiction. Dialogues Clin Neurosci. 2014;16(3):335–44.
Santamaria X, Taylor H. MicroRNA and gynecological reproductive diseases. Fertil Steril. 2014;101(6):1545–51.
Tan L, Yu J-T, Tan L. Causes and consequences of MicroRNA dysregulation in neurodegenerative diseases. Mol Neurobiol. 2015;51(3):1249–62.
Wang C, Ji B, Cheng B, et al. Neuroprotection of microRNA in neurological disorders (review). Biomed Rep. 2014;2(5):611–9.
Henshall DC. MicroRNA and epilepsy: profiling, functions and potential clinical applications. Curr Opin Neurol. 2014;27(2):199–205.
Su W, Aloi MS, Garden GA. MicroRNAs mediating CNS inflammation: small regulators with powerful potential. Brain Behav Immun. 2015;52:1–8.
Xu B, Karayiorgou M, Gogos JA. MicroRNAs in psychiatric and neurodevelopmental disorders. Brain Res. 2010;1338:78–88.
Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39(Database issue):D152–7.
Friedman RC, Farh KK-H, Burge CB, et al. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19(1):92–105.
Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843–54.
Yáñez-Mó M, Siljander PR-M, Andreu Z, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. This recent reviewed written by experts in the field of EVs provides a thorough description of EVs, about their properties, functions, and current or ongoing research for clinical utilization.
Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015;25(6):364–72.
Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89.
Thery C. Exosomes: secreted vesicles and intercellular communications. F1000 Biol Rep. 2011;3(Journal Article):15–5.
Vlassov AV, Magdaleno S, Setterquist R, et al. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820(7):940–8.
Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–9.
Nolte-’t Hoen ENM, Buermans HPJ, Waasdorp M, et al. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 2012;40(18):9272–85.
Mittelbrunn M, Gutiérrez-Vázquez C, Villarroya-Beltri C, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282.
Batagov AO, Kuznetsov VA, Kurochkin IV. Identification of nucleotide patterns enriched in secreted RNAs as putative cis-acting elements targeting them to exosome nano-vesicles. BMC Genomics. 2011;12 Suppl 3:S18.
Kalra H, Simpson RJ, Ji H, et al. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10(12):e1001450.
Mathivanan S, Fahner CJ, Reid GE, et al. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40(Database issue):D1241–4.
D.-K. Kim, B. Kang, O. Y. Kim, et al. EVpedia: an integrated database of high-throughput data for systemic analyses of extracellular vesicles. J Extracell Vesicles 2013;2.
Lopez-Ramirez MA, Nicoli S. Role of miRNAs and epigenetics in neural stem cell fate determination. Epigenetics. 2014;9(1):90–100.
Stappert L, Roese-Koerner B, Brüstle O. The role of microRNAs in human neural stem cells, neuronal differentiation and subtype specification. Cell Tissue Res. 2015;359(1):47–64.
Chivet M, Javalet C, Hemming F, et al. Exosomes as a novel way of interneuronal communication. Biochem Soc Trans. 2013;41(1):241–4.
Frühbeis C, Fröhlich D, Kuo WP, et al. Extracellular vesicles as mediators of neuron-glia communication. Front Cell Neurosci. 2013;7:182.
Sharma P, Schiapparelli L, Cline HT. Exosomes function in cell-cell communication during brain circuit development. Curr Opin Neurobiol. 2013;23(Journal Article):997–1004.
Rajendran L, Bali J, Barr MM, et al. Emerging roles of extracellular vesicles in the nervous system. J Neurosci Off J Soc Neurosci. 2014;34(46):15482–9.
Banigan MG, Kao PF, Kozubek JA, et al. Differential expression of exosomal microRNAs in prefrontal cortices of schizophrenia and bipolar disorder patients. PLoS ONE. 2013;8(1):e48814.
Joshi P, Benussi L, Furlan R, et al. Extracellular vesicles in Alzheimer’s disease: friends or foes? Focus on aβ-vesicle interaction. Int J Mol Sci. 2015;16(3):4800–13.
Grandjean P, Landrigan PJ. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014;13(3):330–8.
Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet Lond Engl. 2006;368(9553):2167–78.
Hill DS, Cabrera R, Wallis Schultz D, et al. Autism-like behavior and epigenetic changes associated with autism as consequences of in utero exposure to environmental pollutants in a mouse model. Behav Neurol. 2015;2015:426263.
Pallocca G, Fabbri M, Sacco MG, et al. miRNA expression profiling in a human stem cell-based model as a tool for developmental neurotoxicity testing. Cell Biol Toxicol. 2013;29(4):239–57.
Nerini-Molteni S, Mennecozzi M, Fabbri M, et al. MicroRNA profiling as a tool for pathway analysis in a human in vitro model for neural development. Curr Med Chem. 2012;19(36):6214–23.
Wang L, Bammler TK, Beyer RP, et al. Copper-induced deregulation of microRNA expression in the zebrafish olfactory system. Environ Sci Technol. 2013;47(13):7466–74.
An J, Cai T, Che H, et al. The changes of miRNA expression in rat hippocampus following chronic lead exposure. Toxicol Lett. 2014;229(1):158–66.
Oh J-H, Son M-Y, Choi M-S, et al. Integrative analysis of genes and miRNA alterations in human embryonic stem cells-derived neural cells after exposure to silver nanoparticles. Toxicol Appl Pharmacol. 2015;299:8–23.
Lukiw WJ, Pogue AI. Induction of specific micro RNA (miRNA) species by ROS-generating metal sulfates in primary human brain cells. J Inorg Biochem. 2007;101(9):1265–9.
Pogue AI, Li YY, Cui J-G, et al. Characterization of an NF-kappaB-regulated, miRNA-146a-mediated down-regulation of complement factor H (CFH) in metal-sulfate-stressed human brain cells. J Inorg Biochem. 2009;103(11):1591–5.
Wang L-L, Zhang Z, Li Q, et al. Ethanol exposure induces differential microRNA and target gene expression and teratogenic effects which can be suppressed by folic acid supplementation. Hum Reprod. 2009;24(3):562–79.
Sathyan P, Golden HB, Miranda RC. Competing interactions between micro-RNAs determine neural progenitor survival and proliferation after ethanol exposure: evidence from an ex vivo model of the fetal cerebral cortical neuroepithelium. J Neurosci Off J Soc Neurosci. 2007;27(32):8546–57.
Tsai P-C, Bake S, Balaraman S, et al. MiR-153 targets the nuclear factor-1 family and protects against teratogenic effects of ethanol exposure in fetal neural stem cells. Biol Open. 2014;3(8):741–58.
Qi Y, Zhang M, Li H, et al. MicroRNA-29b regulates ethanol-induced neuronal apoptosis in the developing cerebellum through SP1/RAX/PKR cascade. J Biol Chem. 2014;289(14):10201–10.
Lippai D, Bala S, Catalano D, et al. Micro-RNA-155 deficiency prevents alcohol-induced serum endotoxin increase and small bowel inflammation in mice. Alcohol Clin Exp Res. 2014;38(8):2217–24.
Pietrzykowski AZ, Friesen RM, Martin GE, et al. Posttranscriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron. 2008;59(2):274–87.
Pappalardo-Carter DL, Balaraman S, Sathyan P, et al. Suppression and epigenetic regulation of MiR-9 contributes to ethanol teratology: evidence from zebrafish and murine fetal neural stem cell models. Alcohol Clin Exp Res. 2013;37(10):1657–67.
Lewohl JM, Nunez YO, Dodd PR, et al. Up-regulation of microRNAs in brain of human alcoholics. Alcohol Clin Exp Res. 2011;35(11):1928–37.
Yadav S, Pandey A, Shukla A, et al. miR-497 and miR-302b regulate ethanol-induced neuronal cell death through BCL2 protein and cyclin D2. J Biol Chem. 2011;286(43):37347–57.
Smirnova L, Block K, Sittka A, et al. MicroRNA profiling as tool for in vitro developmental neurotoxicity testing: the case of sodium valproate. PLoS One. 2014;9(6):e98892.
Aluru N, Deak K, Jenny MJ, et al. Developmental exposure to valproic acid alters the expression of microRNAs involved in neurodevelopment in zebrafish. Neurotoxicol Teratol. 2013;40:46–58.
Chen C-L, Liu H, Guan X. Changes in microRNA expression profile in hippocampus during the acquisition and extinction of cocaine-induced conditioned place preference in rats. J Biomed Sci. 2013;20:96.
Avissar-Whiting M, Veiga KR, Uhl KM, et al. Bisphenol A exposure leads to specific microRNA alterations in placental cells. Reprod Toxicol Elmsford N. 2010;29(4):401–6.
Ogata K, Sumida K, Miyata K, et al. Circulating miR-9* and miR-384-5p as potential indicators for trimethyltin-induced neurotoxicity. Toxicol Pathol. 2015;43(2):198–208.
Maccani MA, Avissar-Whiting M, Banister CE, et al. Maternal cigarette smoking during pregnancy is associated with downregulation of miR-16, miR-21, and miR-146a in the placenta. Epigenetics. 2010;5(7):583–9.
Fossati S, Baccarelli A, Zanobetti A, et al. Ambient particulate air pollution and microRNAs in elderly men. Epidemiol Camb Mass. 2014;25(1):68–78.
Laterza OF, Lim L, Garrett-Engele PW, et al. Plasma microRNAs as sensitive and specific biomarkers of tissue injury. Clin Chem. 2009;55(11):1977–83.
Zhang B, Pan X. DX induces aberrant expression of microRNAs in mouse brain and liver. Environ Health Perspect. 2009;117(2):231–40.
Deng Y, Ai J, Guan X, Wang Z, et al. MicroRNA and messenger RNA profiling reveals new biomarkers and mechanisms for RDX induced neurotoxicity. BMC Genomics. 2014;15 Suppl 11:S1.
Kempf SJ, Casciati A, Buratovic S, et al. The cognitive defects of neonatally irradiated mice are accompanied by changed synaptic plasticity, adult neurogenesis and neuroinflammation. Mol Neurodegener. 2014;9:57.
De Felice B, Manfellotto F, Palumbo A, et al. Genome-wide microRNA expression profiling in placentas from pregnant women exposed to BPA. BMC Med Genomics. 2015;8:56.
Marczylo EL, Amoako AA, Konje JC, et al. Smoking induces differential miRNA expression in human spermatozoa: a potential transgenerational epigenetic concern? Epigenetics. 2012;7(5):432–9.
Bai W, Chen Y, Yang J, et al. Aberrant miRNA profiles associated with chronic benzene poisoning. Exp Mol Pathol. 2014;96(3):426–30.
Tyler CR, Allan AM. The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: a review. Curr Environ Health Rep. 2014;1:132–47.
Rager JE, Bailey KA, Smeester L, et al. Prenatal arsenic exposure and the epigenome: altered microRNAs associated with innate and adaptive immune signaling in newborn cord blood. Environ Mol Mutagen. 2014;55(3):196–208.
Caito S, Aschner M. Neurotoxicity of metals. Handb Clin Neurol. 2015;131:169–89.
Mason LH, Harp JP, Han DY. Pb neurotoxicity: neuropsychological effects of lead toxicity. BioMed Res Int. 2014;2014:840547.
Guilarte TR, Opler M, Pletnikov M. Is lead exposure in early life an environmental risk factor for schizophrenia? Neurobiological connections and testable hypotheses. Neurotoxicology. 2012;33(3):560–74.
Lidsky TI, Schneider JS. Lead neurotoxicity in children: basic mechanisms and clinical correlates. Brain J Neurol. 2003;126(Pt 1):5–19.
Lidsky TI, Schneider JS. Adverse effects of childhood lead poisoning: the clinical neuropsychological perspective. Environ Res. 2006;100(2):284–93.
Solan TD, Lindow SW. Mercury exposure in pregnancy: a review. J Perinat Med. 2014;42(6):725–9.
Bose-O’Reilly S, McCarty KM, Steckling N, et al. Mercury exposure and children’s health. Curr Probl Pediatr Adolesc Health Care. 2010;40(8):186–215.
Taber KH, Hurley RA. Mercury exposure: effects across the lifespan. J Neuropsychiatry Clin Neurosci. 2008;20(4):iv–389.
Miranda RC. MicroRNAs and fetal brain development: implications for ethanol teratology during the second trimester period of neurogenesis. Front Genet. 2012;3:77.
Degroote S, Hunting D, Sébire G, et al. Autistic-like traits in Lewis rats exposed perinatally to a mixture of common endocrine disruptors. Endocr Disruptors. 2014;2(1):e976123.
Wood AG, Nadebaum C, Anderson V, et al. Prospective assessment of autism traits in children exposed to antiepileptic drugs during pregnancy. Epilepsia. 2015;56(7):1047–55.
Velez-Ruiz NJ, Meador KJ. Neurodevelopmental effects of fetal antiepileptic drug exposure. Drug Saf. 2015;38(3):271–8.
Cohen MJ, Meador KJ, Browning N, et al. Fetal antiepileptic drug exposure: adaptive and emotional/behavioral functioning at age 6 years. Epilepsy Behav EB. 2013;29(2):308–15.
P. Ranger and B. A. Ellenbroek. Perinatal influences of valproate on brain and behaviour: An animal model for autism. Curr Top Behav Neurosci 2015.
Glebov K, Löchner M, Jabs R, et al. Serotonin stimulates secretion of exosomes from microglia cells. Glia. 2015;63(4):626–34.
Chivet M, Javalet C, Laulagnier K, et al. Exosomes secreted by cortical neurons upon glutamatergic synapse activation specifically interact with neurons. J Extracell Vesicles. 2014;3:24722.
Brites D, Fernandes A. Neuroinflammation and depression: microglia activation, extracellular microvesicles and microRNA dysregulation. Front Cell Neurosci. 2015;9:476.
Pegtel DM, Peferoen L, Amor S. Extracellular vesicles as modulators of cell-to-cell communication in the healthy and diseased brain. Philos Trans R Soc Lond B Biol Sci. 2014;369:1652.
Fauré J, Lachenal G, Court M, et al. Exosomes are released by cultured cortical neurons. Mol Cell Neurosci. 2006;31(4):642–8.
Krämer-Albers E-M, Bretz N, Tenzer S, et al. Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: trophic support for axons? Proteomics Clin Appl. 2007;1(11):1446–61.
Guescini M, Genedani S, Stocchi V, Agnati LF. Astrocytes and glioblastoma cells release exosomes carrying mtDNA. J Neural Transm Vienna Aust 1996. 2010;117(1):1–4.
Potolicchio I, Carven GJ, Xu X, Stipp C, et al. Proteomic analysis of microglia-derived exosomes: metabolic role of the aminopeptidase CD13 in neuropeptide catabolism. J Immunol Baltim Md 1950. 2005;175(4):2237–43.
Lopez-Verrilli MA, Picou F, Court FA. Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia. 2013;61(11):1795–806.
Grapp M, Wrede A, Schweizer M, et al. Choroid plexus transcytosis and exosome shuttling deliver folate into brain parenchyma. Nat Commun. 2013;4(Journal Article):2123.
Street JM, Barran PE, Mackay CL, et al. Identification and proteomic profiling of exosomes in human cerebrospinal fluid. J Transl Med. 2012;10:5.
Wang G, Dinkins M, He Q, Zhu G, et al. Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4): potential mechanism of apoptosis induction in Alzheimer disease (AD). J Biol Chem. 2012;287(25):21384–95.
Taylor AR, Robinson MB, Gifondorwa DJ, et al. Regulation of heat shock protein 70 release in astrocytes: role of signaling kinases. Dev Neurobiol. 2007;67(13):1815–29.
Frühbeis C, Fröhlich D, Kuo WP, et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 2013;11(7):e1001604.
Bakhti M, Winter C, Simons M. Inhibition of myelin membrane sheath formation by oligodendrocyte-derived exosome-like vesicles. J Biol Chem. 2011;286(1):787–96.
Fröhlich D, Kuo WP, Frühbeis C, et al. Multifaceted effects of oligodendroglial exosomes on neurons: impact on neuronal firing rate, signal transduction and gene regulation. Philos Trans R Soc Lond B Biol Sci. 2014;369:1652.
Pasquinelli AE, Reinhart BJ, Slack F, et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000;408(6808):86–9.
Ziats MN, Rennert OM. Identification of differentially expressed microRNAs across the developing human brain. Mol Psychiatry. 2014;19(7):848–52.
Moreau MP, Bruse SE, Jornsten R, et al. Chronological changes in microRNA expression in the developing human brain. PLoS One. 2013;8(4):e60480.
Babenko O, Kovalchuk I, Metz GAS. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci Biobehav Rev. 2015;48:70–91.
Smirnova L, Gräfe A, Seiler A, et al. Regulation of miRNA expression during neural cell specification. Eur J Neurosci. 2005;21(6):1469–77.
Fiore R, Siegel G, Schratt G. MicroRNA function in neuronal development, plasticity and disease. Biochim Biophys Acta. 2008;1779(8):471–8.
Schaefer A, O’Carroll D, Tan CL, et al. Cerebellar neurodegeneration in the absence of microRNAs. J Exp Med. 2007;204(7):1553–8.
Kataoka Y, Takeichi M, Uemura T. Developmental roles and molecular characterization of a Drosophila homologue of Arabidopsis Argonaute1, the founder of a novel gene superfamily. Genes Cells Devoted Mol Cell Mech. 2001;6(4):313–25.
Giraldez AJ, Cinalli RM, Glasner ME, et al. MicroRNAs regulate brain morphogenesis in zebrafish. Science. 2005;308(5723):833–8.
Huang T, Liu Y, Huang M, et al. Wnt1-cre-mediated conditional loss of Dicer results in malformation of the midbrain and cerebellum and failure of neural crest and dopaminergic differentiation in mice. J Mol Cell Biol. 2010;2(3):152–63.
Hengst U, Cox LJ, Macosko EZ, et al. Functional and selective RNA interference in developing axons and growth cones. J Neurosci. 2006;26(21):5727–32.
Krichevsky AM, Sonntag K-C, Isacson O, et al. Specific microRNAs modulate embryonic stem cell-derived neurogenesis. Stem Cells Dayt Ohio. 2006;24(4):857–64.
Hou Q, Ruan H, Gilbert J, et al. MicroRNA miR124 is required for the expression of homeostatic synaptic plasticity. Nat Commun. 2015;6:10045.
Yoo AS, Sun AX, Li L, et al. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature. 2011;476(7359):228–31.
Feliciano DM, Zhang S, Nasrallah CM, et al. Embryonic cerebrospinal fluid nanovesicles carry evolutionarily conserved molecules and promote neural stem cell amplification. PLoS One. 2014;9(2):e88810.
Sun E, Shi Y. MicroRNAs: small molecules with big roles in neurodevelopment and diseases. Exp Neurol. 2015;268:46–53.
Hui Z, Yongchao Z, Yongqing Z. Recent progresses in molecular genetics of autism spectrum disorders. Yi Chuan Hered Zhongguo Yi Chuan Xue Hui Bian Ji. 2015;37(9):845–54.
Washbourne P. Synapse assembly and neurodevelopmental disorders. Neuropsychopharmacology. 2015;40(1):4–15.
Lachenal G, Pernet-Gallay K, Chivet M, et al. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol Cell Neurosci. 2011;46(2):409–18.
Bak M, Silahtaroglu A, Møller M, et al. MicroRNA expression in the adult mouse central nervous system. RNA. 2008;14(3):432–44.
Kye M-J, Liu T, Levy SF, et al. Somatodendritic microRNAs identified by laser capture and multiplex RT-PCR. RNA N Y N. 2007;13(8):1224–34.
Mellios N, Sugihara H, Castro J, et al. miR-132, an experience-dependent microRNA, is essential for visual cortex plasticity. Nat Neurosci. 2011;14(10):1240–2.
Hansen KF, Karelina K, Sakamoto K, Wayman GA, Impey S, Obrietan K. miRNA-132: a dynamic regulator of cognitive capacity. Brain Struct Funct. 2013;218(3):817–31.
Karpova NN. Role of BDNF epigenetics in activity-dependent neuronal plasticity. Neuropharmacology. 2014;76(Pt C):709–18.
Aksoy-Aksel A, Zampa F, Schratt G. MicroRNAs and synaptic plasticity—a mutual relationship. Philos Trans R Soc Lond B Biol Sci. 2014;369:1652.
Ryan B, Joilin G, Williams JM. Plasticity-related microRNA and their potential contribution to the maintenance of long-term potentiation. Front Mol Neurosci. 2015;8:4.
Earls LR, Westmoreland JJ, Zakharenko SS. Non-coding RNA regulation of synaptic plasticity and memory: implications for aging. Ageing Res Rev. 2014;17:34–42.
Codocedo JF, Inestrosa NC. Environmental control of microRNAs in the nervous system: implications in plasticity and behavior. Neurosci Biobehav Rev. 2016;60:121–38.
Saugstad JA. Non-coding RNAs in stroke and neuroprotection. Front Neurol. 2015;6:50.
Lusardi TA, Farr CD, Faulkner CL, et al. Ischemic preconditioning regulates expression of microRNAs and a predicted target, MeCP2, in mouse cortex. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2010;30(4):744–56.
Sun Y, Luo Z-M, Guo X-M, et al. An updated role of microRNA-124 in central nervous system disorders: a review. Front Cell Neurosci. 2015;9:193.
Abdolmaleky HM, Zhou J-R, Thiagalingam S. An update on the epigenetics of psychotic diseases and autism. Epigenomics. 2015;7(3):427–49.
Miller BH, Wahlestedt C. MicroRNA dysregulation in psychiatric disease. Brain Res. 2010;1338:89–99.
Issler O, Chen A. Determining the role of microRNAs in psychiatric disorders. Nat Rev Neurosci. 2015;16(4):201–12.
Hommers LG, Domschke K, Deckert J. Heterogeneity and individuality: microRNAs in mental disorders. J Neural Transm Vienna Aust 1996. 2015;122(1):79–97.
Zhou R, Yuan P, Wang Y, et al. Evidence for selective microRNAs and their effectors as common long-term targets for the actions of mood stabilizers. Neuropsychopharmacol Off Publ Am Coll Neuropsychopharmacol. 2009;34(6):1395–405.
Beveridge NJ, Gardiner E, Carroll AP, et al. Schizophrenia is associated with an increase in cortical microRNA biogenesis. Mol Psychiatry. 2010;15(12):1176–89.
Nguyen LS, Lepleux M, Makhlouf M, et al. Profiling olfactory stem cells from living patients identifies miRNAs relevant for autism pathophysiology. Mol Autism. 2016;7:1.
Abu-Elneel K, Liu T, Gazzaniga FS, et al. Heterogeneous dysregulation of microRNAs across the autism spectrum. Neurogenetics. 2008;9(3):153–61.
Ander BP, Barger N, Stamova B, et al. Atypical miRNA expression in temporal cortex associated with dysregulation of immune, cell cycle, and other pathways in autism spectrum disorders. Mol Autism. 2015;6:37.
Talebizadeh Z, Butler MG, Theodoro MF. Feasibility and relevance of examining lymphoblastoid cell lines to study role of microRNAs in autism. Autism Res Off J Int Soc Autism Res. 2008;1(4):240–50.
Ghahramani Seno MM, Hu P, Gwadry FG, et al. Gene and miRNA expression profiles in autism spectrum disorders. Brain Res. 2011;1380:85–97.
Sarachana T, Zhou R, Chen G, et al. Investigation of post-transcriptional gene regulatory networks associated with autism spectrum disorders by microRNA expression profiling of lymphoblastoid cell lines. Genome Med. 2010;2(4):23.
Mundalil Vasu M, Anitha A, Thanseem I, et al. Serum microRNA profiles in children with autism. Mol Autism. 2014;5:40.
Huang F, Zhou T, Yao X, et al. miRNA profiling in autism spectrum disorder in China. Genomics Data. 2015;6:108–9.
Banerjee-Basu S, Larsen E, Muend S. Common microRNAs target established ASD genes. Front Neurol. 2014;5:205.
Mellios N, Sur M. The emerging role of microRNAs in schizophrenia and autism spectrum disorders. Front Psych. 2012;3:39.
Olde Loohuis NFM, Kole K, Glennon JC, et al. Elevated microRNA-181c and microRNA-30d levels in the enlarged amygdala of the valproic acid rat model of autism. Neurobiol Dis. 2015;80:42–53.
Kandemir H, Erdal ME, Selek S, et al. Evaluation of several micro RNA (miRNA) levels in children and adolescents with attention deficit hyperactivity disorder. Neurosci Lett. 2014;580:158–62.
Wu LH, Peng M, Yu M, et al. Circulating MicroRNA Let-7d in attention-deficit/hyperactivity disorder. Neuromolecular Med. 2015;17(2):137–46.
Chistiakov DA, Chekhonin VP. Extracellular vesicles shed by glioma cells: pathogenic role and clinical value. Tumour Biol J Int Soc Oncodevelopmental Biol Med. 2014;35(9):8425–38.
Gonda DD, Akers JC, Kim R, et al. Neuro-oncologic applications of exosomes, microvesicles, and other nano-sized extracellular particles. Neurosurgery. 2013;72(4):501–10.
Viaud S, Théry C, Ploix S, et al. Dendritic cell-derived exosomes for cancer immunotherapy: what’s next? Cancer Res. 2010;70(4):1281–5.
Liddelow SA. Development of the choroid plexus and blood-CSF barrier. Front Neurosci. 2015;9:32.
Moretti R, Pansiot J, Bettati D, et al. Blood–brain barrier dysfunction in disorders of the developing brain. Front Neurosci. 2015;9:40.
Alvarez-Erviti L, Seow Y, Yin H, Betts C, et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341–5.
Lakhal S, Wood MJA. Exosome nanotechnology: an emerging paradigm shift in drug delivery: exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers. BioEssays News Rev Mol Cell Dev Biol. 2011;33(10):737–41.
Skog J, Würdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10(12):1470–6.
Wood MJA, O’Loughlin AJ, Samira L. Exosomes and the blood–brain barrier: implications for neurological diseases. Ther Deliv. 2011;2(9):1095–9.
Kapogiannis D, Boxer A, Abner E, et al. Neural origin plasma exosomes provide novel biomarkers for brain insulin resistance in Alzheimer’s disease (I11-5B). Neurology. 2015;84(14 Supplement):I11–5B.
Shao H, Chung J, Balaj L, et al. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat Med. 2012;18(12):1835–40.
Theoharides TC, Zhang B. Neuro-inflammation, blood–brain barrier, seizures and autism. J Neuroinflammation. 2011;8:168.
de Vries HE, Kooij G, Frenkel D, et al. Inflammatory events at blood–brain barrier in neuroinflammatory and neurodegenerative disorders: implications for clinical disease. Epilepsia. 2012;53 Suppl 6:45–52.
Jia R, Li J, Rui C, et al. Comparative proteomic profile of the human umbilical cord blood exosomes between normal and preeclampsia pregnancies with high-resolution mass spectrometry. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2015;36(6):2299–306.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Virginie Gillet, Darel John Hunting, and Larissa Takser declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Topical Collection on Environmental Epigenetics
Rights and permissions
About this article
Cite this article
Gillet, V., Hunting, D.J. & Takser, L. Turing Revisited: Decoding the microRNA Messages in Brain Extracellular Vesicles for Early Detection of Neurodevelopmental Disorders. Curr Envir Health Rpt 3, 188–201 (2016). https://doi.org/10.1007/s40572-016-0093-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40572-016-0093-0