Abstract
Background
Intervention against age-related neurodegenerative diseases may be difficult once extensive structural and functional deteriorations have already occurred in the brain.
Aim
Investigating 6-year longitudinal changes and implications of regional brain atrophy and functional connectivity in the triple-network model as biomarkers of preclinical cognitive impairment in healthy aging.
Methods
We acquired longitudinal cognitive scores and magnetic resonance imaging (MRI) data from 74 healthy old adults. Resting-state functional MRI (rs-fMRI) analysis was conducted using FSL6.0.1 to examine functional connectivity changes and regional brain morphometries were quantified using FreeSurfer5.3. Finally, we cross-validated and compared two support vector machine (SVM) regression models to predict future 6-year cognition score from the baseline regional brain atrophy and resting-state functional connectivity (rs-FC) measures.
Results
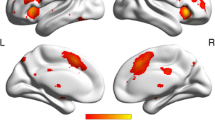
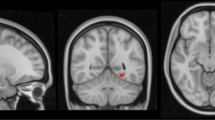
After a 6-year follow-up, our results (P < 0.05-corrected) indicated significant connectivity reduction within all the three brain networks, significant differences in regional brain volumes and cortical thickness. We also observed significant improvement in episodic memory and significant decline in executive functions. Finally, comparing the two models, we observed that regional brain atrophy predictors were more efficient in approximating future 6-year cognitive scores (R = 0.756, P < 0.0001) than rs-FC predictors (R = 0.6, P < 0.0001).
Conclusion
This study used longitudinal data to keep subject variability low and to increase the validity of the results. We demonstrated significant changes in structural and functional MRI over 6 years. Our findings present a potential neuroimaging-based biomarker to detect cognitive impairment and prevent risks of neurodegenerative diseases in healthy old adults.





Similar content being viewed by others
Data availability
The magnetic resonance imaging (MRI) and neuropsychological tests data that support the findings of this study are available in [“OASIS-3”] with the identifier(s) [https://doi.org/10.1101/2019.12.13.19014902] [8]. OASIS-3 data are openly available to the scientific community at https://www.oasisbrains.org. Prior to accessing the data, users are required to agree to the OASIS data use terms (DUT), which follow the creative commons attribution 4.0 license.
References
Sperling R, Mormino E, Johnson K (2014) The evolution of preclinical Alzheimer’s disease: implications for prevention trials. Neuron 84:608–622
Yamada T, Hashimoto R-I, Yahata N et al (2017) Resting-state functional connectivity-based biomarkers and functional MRI-based neurofeedback for psychiatric disorders: a challenge for developing theranostic biomarkers. Int J Neuropsychopharmacol 20:769–781
Hu Z, Wu L, Jia J et al (2014) Advances in longitudinal studies of amnestic mild cognitive impairment and Alzheimer’s disease based on multi-modal MRI techniques. Neurosci Bull 30:198–206
Eavani H, Habes M, Satterthwaite TD et al (2018) Heterogeneity of structural and functional imaging patterns of advanced brain aging revealed via machine learning methods. Neurobiol Aging 71:41–50
Salat DH, Buckner RL, Snyder AZ et al (2004) Thinning of the cerebral cortex in aging. Cereb Cortex 14:721–730
Hafkemeijer A, van der Grond J, Rombouts SARB (2012) Imaging the default mode network in aging and dementia. Biochim Biophys Acta Mol Basis Dis 1822:431–441
Oschmann M, Gawryluk JR (2020) A longitudinal study of changes in resting-state functional magnetic resonance imaging functional connectivity networks during healthy aging. Brain Connect 10:377–384
LaMontagne PJ, Benzinger TLS, Morris JC et al (2019) OASIS-3: longitudinal neuroimaging, clinical, and cognitive dataset for normal aging and Alzheimer disease. medRxiv 7:44
Ciolek CH, Lee SY (2020) Chapter 19—cognitive issues in the older adult. In: Wong AD (ed) RABT-GGPT, 4th edn. Mosby, St. Louis, pp 425–452
Desmond JE, Glover GH (2002) Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. J Neurosci Methods 118:115–128
Weintraub S, Salmon D, Mercaldo N et al (2009) The Alzheimer’s disease centers’ uniform data set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord 23:91
MacPherson SE, Allerhand M, Cox SR et al (2019) Individual differences in cognitive processes underlying trail making test-B performance in old age: the Lothian birth cohort 1936. Intelligence 75:23–32
Worsley KJ (2001) Statistical analysis of activation images. Functional magnetic resonance imaging. Oxford University Press, Oxford
Jenkinson M (2018) fsl_motion_outliers
Zhang Y, Brady M, Smith S (2001) Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging 20:45–57
Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17:143–155
Jenkinson M, Bannister P, Brady M et al (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841
Greve DN, Fischl B (2009) Accurate and robust brain image alignment using boundary-based registration. Neuroimage 48:63–72
Beckmann CF, Jenkinson M, Smith SM (2003) General multilevel linear modeling for group analysis in FMRI. Neuroimage 20:1052–1063
Woolrich MW, Behrens TEJ, Beckmann CF et al (2004) Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage 21:1732–1747
Gorges M, Kunz MS, Müller H-P et al (2020) Longitudinal brain atrophy distribution in advanced Parkinson’s disease: what makes the difference in “cognitive status” converters? Hum Brain Mapp 41:1416–1434
Marstaller L, Williams M, Rich A et al (2015) Aging and large-scale functional networks: white matter integrity, gray matter volume, and functional connectivity in the resting state. Neuroscience 290:369–378
Menon V (2011) Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 15:483–506
Uddin LQ (2015) Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci 16:55–61
Hamilton JP, Etkin A, Furman DJ et al (2012) Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of base line activation and neural response data. Am J Psychiatry 169:693–703
Wiebking C, Bauer A, de Greck M et al (2010) Abnormal body perception and neural activity in the insula in depression: an fMRI study of the depressed “material me.” World J Biol Psychiatry Off J World Fed Soc Biol Psychiatry 11:538–549
Day GS, Farb NAS, Tang-Wai DF et al (2013) Salience network resting-state activity: prediction of frontotemporal dementia progression. JAMA Neurol 70:1249–1253
Sambataro F, Murty VP, Callicott JH et al (2010) Age-related alterations in default mode network: impact on working memory performance. Neurobiol Aging 31:839–852
Onoda K, Ishihara M, Yamaguchi S (2012) Decreased functional connectivity by aging is associated with cognitive decline. J Cogn Neurosci 24:2186–2198
Zhang H-Y, Chen W-X, Jiao Y et al (2014) Selective vulnerability related to aging in large-scale resting brain networks. PLoS ONE 9:e108807
Ghetti S, DeMaster DM, Yonelinas AP et al (2010) Developmental differences in medial temporal lobe function during memory encoding. J Neurosci 30:9548–9556
Giedd JN, Blumenthal J, Jeffries NO et al (1999) Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 2:861–863
Raz N, Lindenberger U, Rodrigue KM et al (2005) Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex 15:1676–1689
Reuter-Lorenz PA, Grady CL, Cabeza R et al (2013) Frontal lobes and aging deterioration and compensation: deterioration and compensation. Oxford University Press, Oxford
Chen AC, Oathes DJ, Chang C et al (2013) Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proc Natl Acad Sci USA 110:19944–19949
Leech R, Sharp DJ (2014) The role of the posterior cingulate cortex in cognition and disease. Brain 137:12–32
Cacciaglia R, Molinuevo JL, Sánchez-Benavides G et al (2018) Episodic memory and executive functions in cognitively healthy individuals display distinct neuroanatomical correlates which are differentially modulated by aging. Hum Brain Mapp 39:4565–4579
Van Petten C (2004) Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia 42:1394–1413
Foster JK, Meikle A, Goodson G et al (1999) The hippocampus and delayed recall: bigger is not necessarily better? Memory 7:715–732
Raichle ME (2015) The brain’s default mode network. Annu Rev Neurosci 38:433–447
Chai XJ, Ofen N, Gabrieli JDE et al (2014) Development of deactivation of the default-mode network during episodic memory formation. Neuroimage 84:932–938
Kim H, Daselaar SM, Cabeza R (2010) Overlapping brain activity between episodic memory encoding and retrieval: roles of the task-positive and task-negative networks. Neuroimage 49:1045–1054
Wilson RS, Beckett LA, Barnes LL et al (2002) Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging 17:179–193
Morse CK (1993) Does variability increase with age? An archival study of cognitive measures. Psychol Aging 8:156–164
Rabbitt P (1993) Does it all go together when it goes? The nineteenth bartlett memorial lecture. Q J Exp Psychol Sect A 46:385–434
Lee S, Zhou X, Gao Y et al (2018) Episodic memory performance in a multi-ethnic longitudinal study of 13,037 elderly. PLoS ONE 13:e0206803
Chadjikyprianou A, Hadjivassiliou M, Papacostas S et al (2021) The neurocognitive study for the aging: longitudinal analysis on the contribution of sex, age, education and APOE ɛ4 on cognitive performance. Front Genet 12:680531
Salthouse TA (2014) Why are there different age relations in cross-sectional and longitudinal comparisons of cognitive functioning? Curr Dir Psychol Sci 23:252–256
Ahlskog JE, Geda YE, Graff-Radford NR et al (2011) Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clin Proc 86:876–884
Shea TB, Remington R (2018) Cognitive improvement in healthy older adults can parallel that of younger adults following lifestyle modification: support for cognitive reserve during aging. J Alzheimer’s Dis Rep 2:201–205
Deng L, Stanley ML, Monge ZA et al (2021) Age-related compensatory reconfiguration of pfc connections during episodic memory retrieval. Cereb Cortex 31:717–730
Liu X, Tosun D, Weiner MW et al (2013) Locally linear embedding (LLE) for MRI based Alzheimer’s disease classification. Neuroimage 83:148–157
Xu L, Liang G, Liao C et al (2018) An efficient classifier for Alzheimer’s disease genes identification. Molecules. https://doi.org/10.3390/molecules23123140
Elliott ML, Knodt AR, Ireland D et al (2020) What is the test-retest reliability of common task-functional MRI measures? New empirical evidence and a meta-analysis. Psychol Sci 31:792–806
Wilson RS, Leurgans SE, Boyle PA et al (2010) Neurodegenerative basis of age-related cognitive decline. Neurology 75:1070–1078
Acknowledgements
Authors acknowledge the free access of OASIS-3 data, openly available to the scientific community at https://www.oasisbrains.org. The funds were provided to the Knight ADRC and KARI by NIH P50AG00561, P30NS09857781, P01AG026276, P01AG003991, R01AG043434, R01AG054567, UL1TR000448, and R01EB009352, and Florbetapir doses provided by Avid Radiopharmaceuticals, a wholly owned subsidiary of Eli Lilly.
Funding
This work was supported by the National Natural Science Foundation of China (Grant number: 81701665), Chinese Academy of Sciences (block grants) and the World Academy of Sciences (block grants).
Author information
Authors and Affiliations
Contributions
All authors contributed significantly and agreed with the content of the manuscript. Details about author contributions are as follows: conceptualization, data acquisition, methodology, and formal analysis: JdDU. Quality control (QC), and writing—review & editing: BAN. Quality control (QC), validation, and writing—review & editing: YW. Quality control (QC), and writing—review & editing: DZ. Quality control (QC), and writing—review & editing: YL. Quality control (QC), and writing—review & editing: ZJ. Supervision, resources, project administration, and funding acquisition: XW. Supervision, resources, project administration, and funding acquisition: BQ.
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethics approval
The authors state that all applicable institutional and/or national protocols for the human research project were followed and that they conform to the provisions of the Declaration of Helsinki.
Statement of human and animal rights
All human and animal studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
de Dieu Uwisengeyimana, J., Nguchu, B.A., Wang, Y. et al. Longitudinal resting-state functional connectivity and regional brain atrophy-based biomarkers of preclinical cognitive impairment in healthy old adults. Aging Clin Exp Res 34, 1303–1313 (2022). https://doi.org/10.1007/s40520-021-02067-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40520-021-02067-8