Abstract
Purpose of Review
Kidney disease affects more than 13% of the world population, and current treatment options are limited to dialysis and organ transplantation. The generation of kidney organoids from human-induced pluripotent stem (hiPS) cells could be harnessed to engineer artificial organs and help overcome the challenges associated with the limited supply of transplantable kidneys. The purpose of this article is to review the progress in kidney organoid generation and transplantation and highlight some existing challenges in the field. We also examined possible improvements that could help realize the potential of organoids as artificial organs or alternatives for kidney transplantation therapy.
Recent Findings
Organoids are useful for understanding the mechanisms of kidney development, and they provide robust platforms for drug screening, disease modeling, and generation of tissues for organ replacement therapies. Efforts to design organoids rely on the ability of cells to self-assemble and pattern themselves into recognizable tissues. While existing protocols for generating organoids result in multicellular structures reminiscent of the developing kidney, many do not yet fully recapitulate the complex cellular composition, structure, and functions of the intact kidney. Recent advances toward achieving these goals include identifying cell culture conditions that produce organoids with improved vasculature and cell maturation and functional states. Still, additional improvements are needed to enhance tissue patterning, specialization, and function, and avoid tumorigenicity after transplantation.
Summary
This report focuses on kidney organoid studies, advancements and limitations, and future directions for improvements towards transplantation.
Similar content being viewed by others
Introduction
Chronic kidney disease (CKD) is a prevalent global health problem with significant economic and social burdens exacerbated by the recent COVID-19 pandemic [1,2,3,4]. Millions of affected patients die due to limited therapeutic options and the lack of affordable treatments [5]. As such, many patients with CKD progress to end-stage kidney disease (ESKD), requiring either hemodialysis or kidney transplantation for survival. Thus, more efficient and effective therapeutic options are urgently needed to combat the continuing global increase and poor prognosis of CKD. The prospect of using stem cell-derived organoids for kidney replacement therapy could offer an unlimited source of transplantable tissues, which can help address problems related to the shortage of viable organs. Achieving this goal would be one of the most innovative applications of organoid technology in the biomedical and clinical sciences.
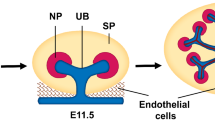
Organoids are spheroids composed of multiple cell types with tissue-like structures that often exhibit features of early organ development based on tissue morphology and patterning, cellular composition, and functional characteristics [6, 7]. Organoids can be developed from pluripotent (Fig. 1) or tissue-specific stem cells and progenitor cells capable of self-organizing and patterning into desired tissue types [8, 9]. Three-dimensional (3D) cell culture technologies are helping to provide additional guidance for cellular organization and produce organoids that recapitulate essential structural and functional properties of human organs, including the brain, kidney, lungs, retina, etc. [8]. Kidney organoids are characterized by the presence of specific kidney and stromal cells [10]. As such, kidney organoids are useful in many applications aimed at understanding disease pathogenesis and improving therapeutic strategies. However, technical limitations (discussed below) have impeded progress in clinical translation. These challenges present opportunities for bioengineers and biologists to uncover new strategies and methods for advancing the field.
In contrast to animal models, organoids are amenable to real-time imaging techniques, allowing experimental manipulation to study developmental processes and molecular events linked to pathologies. For example, kidney organoids offer an advantage over animal models for drug screening applications since organoids are suitable for assays requiring high-throughput formats or handling. Additionally, animal models are insufficient for full recapitulation of human physiology and pathophysiology [11]. Thus, human stem cell-derived organoids are useful for physiologically relevant modeling of disease mechanisms, cell–cell communication, and tissue-tissue interfaces. Traditional cell culture systems often preclude the study of cell behaviors linked to the 3D organization of tissues and organs [9], but such studies are feasible in organoids.
Furthermore, organoids can respond to stress by expressing or releasing injury biomarkers from specific cell populations, making them highly useful for toxicity studies, and drug screening and disease modeling [9, 10, 12, 13]. Notably, primary patient-derived kidney cells, such as human podocytes are difficult to acquire, which underscores the frequent use of animal models to study human disease mechanism, including those associated with mutations found in humans but bot in lab animals such as mice (e.g., APOL-1 and its high-risk variants). Indeed, there is mounting evidence that existing animal models do not faithfully replicate human phenotypes or pathophysiology. Additionally, immortalized podocyte cell lines lack important mature podocyte markers and have decreased functional capacity and reduced sensitivity to doxorubicin (a known nephrotoxin) compared to human iPSC-derived podocytes [14,15,16].
Recent advances in bioengineering technologies have given rise to improved methodologies in organoid research and ex vivo kidney modeling (Fig. 1). To effectively use kidney organoids for regenerative purposes in vivo, the issues of safety, cell maturation, and tissue functionality must be addressed. A key goal in this area is to develop kidney organoids that recapitulate in vivo kidney phenotype and physiology, thereby providing a platform for personalized medicine and transplantation.
Transforming Stem Cells into Kidney Organoids
The kidney contains more than 20 unique cell types, and, like the brain, it exhibits high anatomical complexity when compared to other human organs [8, 17, 18]. During kidney organogenesis, cells of the primitive streak give rise to the mesoderm, which then differentiates to form the intermediate mesoderm (IM). The medio-posterior IM produces the ureteric epithelium and the metanephric mesenchyme, which are further specialized into the collecting ducts and nephron, respectively [19,20,21,22,23]. Studying tissue and organ biology in mammals can be challenging due to ethical concerns and limited access to samples. However, recent advances in stem cell biology have afforded extraordinary opportunities to model kidney development and disease using 2D [15, 24] or 3D [17, 25] cell culture systems. When provided appropriate biochemical and biophysical cues, stem cells can proliferate and form organoids that recapitulate some of the multicellular composition and early stages of tissue development [19, 25]. These features make kidney organoids important models of organ development and disease, with implications for fundamental studies of human biology, drug discovery, and regenerative medicine.
Applications of Kidney Organoids
By using organoids, researchers have established human cellular systems that model disease phenotypes with efficiency and specificity [5]. For example, kidney organoids have been used to model kidney diseases, including polycystic kidney disease (PKD) [10, 26] and proteinopathies such as Mucin 1 kidney disease (MKD) [27, 28]. Additionally, organoids have been used to study podocytopathies, including the pathogenesis of congenital nephrotic syndrome caused by nephrin (NPHS1) mutations [29,30,31]. Organoids can help unravel the complex cellular and molecular events that underlie disease pathogenesis, which can help identify novel therapeutic targets for kidney disease. For instance, Kim et al. used organoids to study the role of podocalyxin (PODXL) during organogenesis to further understand its role in developmental defects [29]. The authors found that PODXL has a conserved and essential role in podocyte maturation. Together, these studies highlight the utility of kidney organoids for studying developmental processes, a result that could not be fully achieved using traditional cell culture models.
The indication that organoids can be used to model human pathologies has inspired studies to explore the possibility of using these in vitro models for drug testing and screening applications [9, 32]. Another vital application of kidney organoids is their use in screening potential toxins, particularly molecules targeting the tubular and glomerular tissues. Organoids can also be used to simultaneously evaluate responses from multiple cell types and subsequent intracellular responses after nephrotoxic injury. Drugs targeting specific kidney compartments have been studied using organoids, e.g., cisplatin which shows specific toxicity for proximal tubular cells [10, 12]. Interestingly, the effect of nephrotoxins on renal cells can be used to differentiate cells based on phenotypical (functional and structural) characteristics and differentiation states [5]. Given that a significant number of new drug candidates fail in clinical trials due to kidney toxicity in humans [33], using kidney organoids for preclinical toxicity studies could strengthen the likelihood of success for drug candidates and save time and reduce the costs of drug development.
Current Challenges in Patterning Kidney Organoids
The kidney is responsible for blood filtration in the body [34], and urine excretion helps maintain homeostasis by removing toxins and waste [35], making the ureter an essential structure in human physiology. Several challenges must be addressed in order to realize the full potential of kidney organoids. One limitation of established kidney organoid protocols is the lack of ureteric bud (the precursor of the kidney’s collecting duct) [34]. This challenge is compounded by the absence of proper vascular units [36] in most renal organoids, and this is particularly concerning given that the kidney receives 20–25% of the cardiac output [34]. The lack of a proper glomerular basement membrane (GBM) impedes studies of the kidney’s filtration barrier, which require tissue-tissue interfaces seen in vivo but mostly nonexistent or adequately developed in kidney organoids [37••, 38]. The GBM, along with the glomerular epithelium and endothelium, are important structures in the kidney glomerulus essential for blood filtration and molecular reabsorption functions. The maturation state of the cells in organoids in vitro is another complication—genetic profiling revealed that most protocols yield organoids that resemble the first or second trimester of human fetal development [34, 39]. Additionally, the nephron-like structures in kidney organoids do not scale up well compared to the in vivo organ. Specifically, each functional kidney contains about 1.5 to 2 million nephrons, but kidney organoids contain about 100 or fewer cell clusters resembling nephron components [40]. Thus, current methods for generating kidney organoids fail to develop functional units necessary for modeling the biology and disease mechanisms occurring in the postnatal or adult organ.
Kidney organoids are cellularly complex, as revealed by single-cell RNA sequencing data confirming the presence of multiple cell types [41]. Intriguingly, nearly all methods (to date) for generating kidney organoids also produce many off-target, non-renal cell populations such as neurons and muscle cells [17, 41]. This undesired heterogeneity problem could potentially result from the use of inadequate cell culture conditions, including the selection and timing of growth factors, or the type of extracellular matrices employed. Compared to the cellular complexity of the kidney, organoids fail to recapitulate renal cell diversity [40]. Notably, stromal cells—which play important roles in kidney development processes such as nephron elongation—are often nonexistent or functional in kidney organoids [40]. The suboptimal composition of cells may underlie the difficulties in maintaining long-term cultures and maturation of kidney organoids [35, 40]. Additionally, organoids tend to become fibrotic, lose nephrons, and exhibit undesired proliferation of non-renal cells when cultured long-term [40]. Extending the culture period in vitro did not improve maturation [37••]. Some studies report that extended in vitro culture worsened differentiation as indicated by the loss of endothelial cells and reduced expression of differentiation markers accompanied by an increase in off-target muscle cell clusters [41].
Other important cell types often missing in kidney organoids include immune cells, which are critical components for studying inflammatory processes [5]. Immune cells help regulate kidney homeostasis and produce biomarkers of disease [42], which make them essential for fully recapitulating renal pathologies in vitro. Additionally, most organoids are propagated on microenvironments that lack mechanical cues present in the native tissue. In the absence of environmental signals that help with development, most renal organoids have limited 3D organization, and physiologically necessary structures such as the slit diaphragm and fluidic circuits are often absent or poorly developed [36]. Technical issues have been attributed to cell heterogeneity (presence of non-renal or undesired cell types), irregular self-organization patterns, and batch-to-batch variations in organoid differentiation [43]. New technologies in bioengineering could help overcome these limitations in the future.
Emerging Technologies to Improve Kidney Organoids Structure and Function
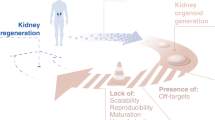
Although patient-derived organoids exhibit substantial variability, they could eventually become useful for personalized medicine applications [9, 26]. A major prospect for organoids is their potential use as transplantable organs (Fig. 2A). The possibility of creating patient specific or molecularly matched kidney organoids with highly developed and functional characteristics could someday minimize the wait time for traditional organ transplantation. This strategy can offer autologous cells and tissues that can be implanted in the form of molds or patches to help recuperate failing renal performance and simultaneously avoid immune rejection.
Applications of kidney organoids. A The present applications of kidney organoids are shown with a potential application involving regenerative medicine and transplantation. B The improvement toward transplantation requires approaches that will take organoid technology from fundamental research to actual translation (transplantable tissues). This figure was created with BiorenderTM
Although a major goal is to use kidney organoids for organ replacement therapy in the future, clinical use of kidney organoids is yet to be achieved. However, some notable progress has been made towards this goal. Inclusion of supportive cell types, such as stromal cells, might encourage additional kidney cell fate specification and maturation [13]. To achieve this, successful manipulation of intrinsic and extrinsic molecular cues and mechanical forces may be necessary to facilitate the development of in vivo-like tissues. Engineered systems that enable nutrient distribution and oxygenation supply could also help enhance organoid viability and functional maturation. Promisingly, hydrogels provide excellent prospects in the development of kidney organoids to address the lack of precise biophysical signals that imitate in vivo biomechanics. This type of scaffold allows for direct modulation of physical factors such as stiffness and porosity, as well as a chemical composition that influences extracellular matrix (ECM) deposition by cells [35]. Hydrogels are excellent biomaterials for guiding cell proliferation, differentiation, and migration, and their properties can be altered to suit the chemical and biophysical cues needed to influence cell fate decisions [44]. They can therefore be used to design appropriate microenvironments for the derivation of kidney organoid. Using hydrogels that simulate biomechanical cues experienced in vivo could drive improved maturation and guide cell fate and minimize the development of off-target populations [35].
Microfluidic devices can also be coupled with bioactive hydrogels (such as those functionalized with ECM proteins or derived from naturally occurring polymers) that facilitate cell adhesion and differentiation [44]. Recent studies by Kim et al. have shown the potential of applying ECM from decellularized animal tissue or primary human kidney tissues in combination with hydrogels to best simulate the molecular cues necessary for renal development [45]. Specifically, the authors created hydrogels from decellularized porcine kidney ECM, in which they differentiated hiPS cells and observed that the matrix-supported growth, maturation, and vascularization of kidney organoids in vitro and in vivo. Another strategy that employed multiple bioengineering technologies is the creation of vascularized hydrogels via bioprinting, which can be specifically engineered to deliver molecular cues to cells and be integrated into organs-on-chips platforms [44]. Lawlor et al. performed extrusion-based bioprinting using hiPS cells which resulted in a more mature kidney organoid development [46]. Although the organoids still lacked vasculature, the authors suggested that this high-throughput technique was reproducible based on cell viability, and yielded large sample sizes while minimizing factors that could introduce variations such as manual manipulations.
To address the limitations associated with the development of fibrotic tissues in long-term cultured organoids, Geuens et al. encapsulated organoids in soft hydrogels derived from natural biocompatible and biodegradable materials [47]. They observed that the fibrotic phenotype was decreased when kidney organoids were encapsulated in the hydrogel compared to the more traditional methods of air–liquid interfaces used by many protocols. The thiol-ene cross-linked alginate hydrogel reduced the abnormal type 1-alpha-1 collagen deposition (associated with renal fibrosis), which they confirmed by proteomic analysis, to be present in prolonged kidney organoid cultures [47]. It is widely recognized that protocols for generating kidney organoids also result in the presence of off-target cells like neurons. Wu et al. discovered that inhibiting brain-derived neurotrophic factor (BDNF) signaling reduced off-target neural cells. Using K252a, an inhibitor of BDNF-NTRK2 (neurotrophic tyrosine kinase receptor type 2) signaling, showed a 90% reduction in neural cell population within kidney organoids [41].
Combining current and emerging understanding of cell differentiation protocols with advances in bioengineering will improve the ability of researchers to develop new and improved methods to generate kidney organoids with the desired cell and tissue differentiation, maturation, patterning, and diversity. Undoubtedly, more rigorous research must be done to advance fundamental research to the actual translation or transplantable tissues. Once these limitations have been addressed, current hiPS cell-derived organoid protocols can be engineered into “mini-kidneys” and potentially into transplantable kidneys for organ replacement therapy in the future.
The Current State of Kidney Organoid Transplantation
Overview of Significant Protocols [37••, 48•, 49••, 50••, 51•, 52••]
Organoids are a promising source of transplantable tissues and functional cell types for cell therapy in regenerative medicine. Such applications have been explored in animal models of retinal degeneration [53,54,55] and colon regeneration [56, 57]. However, organoid transplantation is yet to be achieved for kidney regeneration or injury repair. Achieving this goal in renal medicine relies on the ability to generate organoids that constitute cells capable of reconstructing organ function and possibly providing a niche that shields the graft from damaging or unwanted factors within the cellular microenvironment [9].
Over the past few years, several protocols have been developed to generate and transplant kidney organoids in vivo. We summarized a few of these studies and highlighted their significance in Table 1. We also postulate on how these protocols may help advance the field.
Vascularization of organoids is a critical step toward transplantation given the need for these models to form connections with the host circulatory system. To corroborate that functional vascularization is required for progressive morphogenesis of human kidney organoids, van den Berg et al. developed kidney organoids using a modified version of the protocol first developed by Takasato et al. [17] and then transplanted the resulting organoids into the renal capsule of immunocompromised mice. Although prolonged in vitro culture did not facilitate further differentiation of the kidney organoids, in vivo transplantation promoted the development of vascularized structures. Host-derived vascular networks invaded the transplanted organoids, leading to progressive maturation of tissues resembling the glomerular filtration barrier (GFB) with putative cell-deposited glomerular basement membrane (GBM). The transplanted organoids were also shown to develop glomerular endothelium with fenestrae-like characteristics, as well as apical-basal polarization of the podocytes. After transplantation, the kidney organoids were shown to secrete VEGF and uptake dextran in the proximal tubular cells. By performing glomerular filtration assays in vivo, the authors reported that the human pluripotent stem cell-derived glomeruli could perform barrier functions and discriminate between molecules of varying sizes [37••, 48•].
In another study, Bantounas et al. first obtained kidney progenitor cells using the method previously established by Takasato et al. and then implanted the cells subcutaneously into immunodeficient mice. These implanted cells formed organ-like masses detectable by bioluminescence imaging. Transmission electron microscopy (TEM) also revealed that glomeruli in the implants acquired a distinctive arrangement of podocytes on the outer surface of blood capillaries [50••, 51•].
Although kidney organoids typically lack proper vasculature, Garreta et al. used a different strategy for delivering a vascular network to kidney organoids [49••]. Specifically, their approach involved use of chick chorioallantoic membrane (CAM), a highly vascularized extraembryonic tissue, to create a mechanically compliant in vivo microenvironment that promoted the differentiation and growth of the implanted kidney organoid. CAM is a naturally immunodeficient structure that also provides minimally invasive access to the assay site and therefore facilitate experimental monitoring in situ. CAM blood vessels were developed throughout the kidney organoids within three days after transplantation. Additionally, organoids implanted in the CAM microenvironment showed glomeruli with enlarged Bowman’s space and tube-like structures with enlarged lumens, and CAM blood vessels were present close to the glomerulus. It was shown that soft hydrogels promoted the expression of genes associated with embryonic and mesodermal differentiation and enhanced the formation of kidney organoids. Organoids generated on soft hydrogels (1 kPa) were implanted into CAM for 5 days and displayed better differentiation features when compared with those formed under more rigid conditions (60 kPa hydrogels). The induced podocyte-like cells showed secondary cell processes with slit diaphragm-like structures [49••].
A recent study demonstrated that nephron formation is not a single event in time but occurs through the connection of differentiating epithelial cells and cells derived from the progenitor population, signifying a prerequisite for asynchronous cell populations for tissue formation [58]. Kumar Gupta et al. applied this knowledge in vitro by mixing cells from different stages of differentiation. Using a kidney organoid protocol first developed by Morizane et al. [12], the authors combined cells differentiated at different time points to form heterochronic organoids. Asynchronous mixing of the cells promoted nephrogenesis, and the resulting heterochronic organoids were highly vascularized when implanted under the kidney capsule. Two weeks later, the engrafted kidney tissue was connected to the systemic circulation, and proximal tubule glucose uptake was confirmed as one of the functional readouts. Additionally, the authors indicated that differentiation in organoids is most efficient when the heterochronic mixture of cells was employed [52••].
Together, these protocols demonstrate that transplanted organoids show more maturity than those cultured in vitro alone, as indicated by their enhanced ability to recruit and connect with host vasculature, form glomerulus-like structures, and perform glomerular filtration.
Future Directions and Conclusions
Kidney organoids have the potential to advance renal replacement methods in the future. Strategies have been proposed to address persisting challenges and achieve higher-order kidney organoids. Robotic systems and bioprinting platforms are helping to minimize batch-to-batch variations and develop more consistent organoid differentiation and tissue patterning. Bioprinting technologies could be applied to initially create highly reproducible and consistent small renal units, which could then be assembled to form relevant physiological structures for high-throughput drug screening and potentially for transplantable conduits. Next-generation bioengineering methods can be applied to control organoid differentiation and possibly produce a more accurate and reproducible tissue architecture with precise cellular compositions. By combining bioengineering techniques (e.g., bioprinting) with mathematical models, researchers might be able to adequately provide the necessary biochemical, mechanical, and physical cues to minimize variability in kidney organoids (Fig. 2B). Integration of hydrogel scaffolds and organ-on-a-chip microphysiological systems to support organoid culture in the presence of fluid flow (shear stress) and mechanical cues could also be useful to minimize some or several of the limitations discussed above (Fig. 2B). In the future, advances in organoid research could enable injection of hiPS cells into blastocysts or at the nephrogenic phase in kidney deficient animals to induce generation of large-scale kidneys [43]. Animals such as pigs offer the benefits of growing kidneys of similar size and structure to humans. Wu et al. showed that naïve human pluripotent stem cells engrafted in both pig and cattle pre-implantation blastocysts but display limited contribution to post-implantation pig embryos [59]. Such studies could provide opportunities to generate whole human kidneys to circumvent the shortage of organs for transplantation (Fig. 2B). Substantial amount of research is still required to facilitate the development and implementation of humanized animal models. However, there are concerns with this approach, such as immune rejection, where all renal cell lineages must be removed from the animal due to cross-species transmissions of viruses. Dispersion of transplanted human cells to off-target organs in animal models raises technical and ethical issues [6, 60]. Finally, the use of organoids for regenerative medicine could be combined with in vitro genetic correction strategies like CRISPR/Cas9 genome technology (Fig. 1). This will generate hiPS cell lines representing a wide range of kidney diseases and cell types for the replacement of tissues affected by genetic disorders. The disease-causing mutation can be corrected and with genetic reporters, the effect of the corresponding protein can be studied.
CKD is a prevalent health problem worldwide, with increasing occurrence every year, having severe economic burdens [61] and limited therapies. Kidney organoid research has undoubtedly progressed significantly in the last few years. With new platforms and materials continuously being investigated and developed or improved, we are getting closer to kidney regeneration and precision medicine. Platforms such as hydrogels, bioreactors, 3D printers, and organ-on-chip offer outstanding prospects to improve kidney disease modeling, drug screening, and study kidney development. Large-scale development of transplantable kidney tissues could allow rescuing renal function in patients before it becomes fatal. Current kidney organoid protocols have developed at a fast rate, with every study getting closer to the regenerative kidney goal. Clinical transplantation of organoid-derived cells and tissues might not have been achieved yet, but there is promising evidence that it could be feasible in the future.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Lv JC, Zhang LX. Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol. 2019;1165:3–15.
Kalejaiye TD, Bhattacharya R, Burt MA, Travieso T, Okafor AE, Mou X, et al. SARS-CoV-2 employ BSG/CD147 and ACE2 receptors to directly infect human induced pluripotent stem cell-derived kidney podocytes. Front Cell Dev Biol. 2022;10. https://doi.org/10.3389/fcell.2022.855340.
Musah S. Uncovering SARS-CoV-2 kidney tropism. Nat Rev Mol Cell Biol. 2021;22(8):509. https://doi.org/10.1038/s41580-021-00370-w.
Savedchuk S, Raslan R, Nystrom S, Sparks MA. Emerging viral infections and the potential impact on hypertension, cardiovascular disease, and kidney disease. Circ Res. 2022;130(10):1618–41.
Romero-Guevara R, Ioannides A, Xinaris C. Kidney organoids as disease models: strengths, weaknesses and perspectives. Front Physiol. 2020;11:1384.
Garreta E, Nauryzgaliyeva Z, Montserrat N. Human induced pluripotent stem cell-derived kidney organoids toward clinical implementations. Curr Opin Biomed Eng. 2021;20:100346.
Li M, Izpisua Belmonte JC. Organoids — preclinical models of human disease. N Engl J Med. 2019;380(6):569–79.
Clevers H. Modeling development and disease with organoids. Cell. 2016;165(7):1586–97.
Rossi G, Manfrin A, Lutolf MP. Progress and potential in organoid research. Nat Rev Genet. 2018;19(11):671–87.
Freedman BS, Brooks CR, Lam AQ, Fu H, Morizane R, Agrawal V, et al. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun. 2015;6:8715.
Cheval L, Pierrat F, Rajerison R, Piquemal D, Doucet A. Of mice and men: divergence of gene expression patterns in kidney. PloS one. 2012;7(10):e46876. https://doi.org/10.1371/journal.pone.0046876.
Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV. Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol. 2015;33(11):1193.
Xinaris C. Organoids for replacement therapy: expectations, limitations and reality. Curr Opin Organ Transplant. 2019;24(5):555–61.
Yoshimura Y, Taguchi A, Tanigawa S, Yatsuda J, Kamba T, Takahashi S, et al. Manipulation of nephron-patterning signals enables selective induction of podocytes from human pluripotent stem cells. J Am Soc Nephrol. 2019;30(2):304–21.
Musah S, Mammoto A, Ferrante TC, Jeanty SS, Hirano-Kobayashi M, Mammoto T, et al. Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat Biomed Eng. 2017;1(5):1–12.
Rauch C, Feifel E, Kern G, Murphy C, Meier F, Parson W, et al. Differentiation of human iPSCs into functional podocytes. PLoS One. 2018;13(9):e0203869.
Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature. 2015;526(7574):564–8.
Lee JW, Chou C-L, Knepper MA. Deep sequencing in microdissected renal tubules identifies nephron segment–specific transcriptomes. J Am Soc Nephrol. 2015;26(11):2669–77.
Bhattacharya R, Bonner MG, Musah S. Harnessing developmental plasticity to pattern kidney organoids. Cell Stem Cell. 2021;28(4):587–9.
Kalejaiye TD, Holmes JA, Bhattacharya R, Musah S. Chapter 24 - reconstitution of the kidney glomerular capillary wall. In: Goligorsky MS, editor. Regenerative nephrology. 2nd ed. Academic Press; 2022. p. 331–51.
Xu J, Wong EY, Cheng C, Li J, Sharkar MT, Xu CY, et al. Eya1 interacts with Six2 and Myc to regulate expansion of the nephron progenitor pool during nephrogenesis. Dev Cell. 2014;31(4):434–47.
Taguchi A, Kaku Y, Ohmori T, Sharmin S, Ogawa M, Sasaki H, et al. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell. 2014;14(1):53–67.
Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, et al. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008;3(2):169–81.
Xia Y, Nivet E, Sancho-Martinez I, Gallegos T, Suzuki K, Okamura D, et al. Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nat Cell Biol. 2013;15(12):1507–15.
Howden SE, Wilson SB, Groenewegen E, Starks L, Forbes TA, Tan KS, et al. Plasticity of distal nephron epithelia from human kidney organoids enables the induction of ureteric tip and stalk. Cell Stem Cell. 2021;28(4):671–684. e6.
Cruz NM, Song X, Czerniecki SM, Gulieva RE, Churchill Angela J, Kim YK, et al. Organoid cystogenesis reveals a critical role of microenvironment in human polycystic kidney disease. Nat Mater. 2017;16(11):1112–1119.
Kirby A, Gnirke A, Jaffe DB, Barešová V, Pochet N, Blumenstiel B, et al. Mutations causing medullary cystic kidney disease type 1 lie in a large VNTR in MUC1 missed by massively parallel sequencing. Nat Genet. 2013;45(3):299–303.
Dvela-Levitt M, Kost-Alimova M, Emani M, Kohnert E, Thompson R, Sidhom E-H, et al. Small molecule targets TMED9 and promotes lysosomal degradation to reverse proteinopathy. Cell. 2019;178(3):521–535. e23.
Tanigawa S, Islam M, Sharmin S, Naganuma H, Yoshimura Y, Haque F, et al. Organoids from nephrotic disease-derived iPSCs identify impaired NEPHRIN localization and slit diaphragm formation in kidney podocytes. Stem Cell Rep. 2018;11(3):727–40.
Hale LJ, Howden SE, Phipson B, Lonsdale A, Er PX, Ghobrial I, et al. 3D organoid-derived human glomeruli for personalised podocyte disease modelling and drug screening. Nat Commun. 2018;9(1):5167.
Kim YK, Refaeli I, Brooks CR, Jing P, Gulieva RE, Hughes MR, et al. Gene-edited human kidney organoids reveal mechanisms of disease in podocyte development. Stem cells. 2017;35(12):2366–78.
Wu H, Huang J. Drug-induced nephrotoxicity: pathogenic mechanisms, biomarkers and prevention strategies. Curr Drug Metab. 2018;19(7):559–67. https://doi.org/10.2174/1389200218666171108154419.
Yin L, Du G, Zhang B, Zhang H, Yin R, Zhang W, et al. Efficient drug screening and nephrotoxicity assessment on co-culture microfluidic kidney chip. Sci Rep. 2020;10(1):1–11.
Islam M, Nishinakamura R. How to rebuild the kidney: recent advances in kidney organoids. J Biochem. 2019;166(1):7–12.
Geuens T, van Blitterswijk CA, LaPointe VL. Overcoming kidney organoid challenges for regenerative medicine. NPJ Regen Med. 2020;5(1):1–6.
Koning M, van den Berg CW, Rabelink TJ. Stem cell-derived kidney organoids: engineering the vasculature. Cell Mol Life Sci. 2020;77(12):2257–73.
van den Berg CW, Ritsma L, Avramut MC, Wiersma LE, van den Berg BM, Leuning DG, et al. Renal subcapsular transplantation of PSC-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo. Stem Cell Rep. 2018;10(3):751–765. Established that vascularization is required for progressive morphogenesis of human kidney organoids and confirmed that prolonged (more than 32 days) in vitro culture does not facilitate further differentiation of kidney organoids.
Scott RP, Quaggin SE. The cell biology of renal filtration. J Cell Biol. 2015;209(2):199–210.
Khoshdel-Rad N, Ahmadi A, Moghadasali R. Kidney organoids: current knowledge and future directions. Cell Tissue Res. 2022;387(2):207–24. https://doi.org/10.1007/s00441-021-03565-x.
Little MH, Combes AN. Kidney organoids: accurate models or fortunate accidents. Genes Dev. 2019;33(19–20):1319–45.
Wu H, Uchimura K, Donnelly EL, Kirita Y, Morris SA, Humphreys BD. Comparative analysis and refinement of human PSC-derived kidney organoid differentiation with single-cell transcriptomics. Cell Stem Cell. 2018;23(6):869–881. e8.
Tecklenborg J, Clayton D, Siebert S, Coley S. The role of the immune system in kidney disease. Clin Exp Immunol. 2018;192(2):142–50.
Yousef Yengej FA, Jansen J, Rookmaaker MB, Verhaar MC, Clevers H. Kidney organoids and tubuloids. Cells. 2020;9(6):1326.
Liu H, Wang Y, Cui K, Guo Y, Zhang X, Qin J. Advances in hydrogels in organoids and organs-on-a-chip. Adv Mater. 2019;31(50):1902042.
Kim JW, Nam SA, Yi J, Kim JY, Lee JY, Park S-Y, et al. Kidney decellularized extracellular matrix enhanced the vascularization and maturation of human kidney organoids. Adv Sci. 2022;9(15):2103526. https://doi.org/10.1002/advs.202103526.
Lawlor KT, Vanslambrouck JM, Higgins JW, Chambon A, Bishard K, Arndt D, et al. Cellular extrusion bioprinting improves kidney organoid reproducibility and conformation. Nat Mater. 2021;20(2):260–71.
Geuens T, Ruiter FA, Schumacher A, Morgan FL, Rademakers T, Wiersma LE, et al. Thiol-ene cross-linked alginate hydrogel encapsulation modulates the extracellular matrix of kidney organoids by reducing abnormal type 1a1 collagen deposition. Biomaterials. 2021;275:120976.
van den Berg CW, Koudijs A, Ritsma L, Rabelink TJ. In vivo assessment of size-selective glomerular sieving in transplanted human induced pluripotent stem cell–derived kidney organoids. J Am Soc Nephrol. 2020:31(5):921–929. Demonstrated that engrafted organoid can perform in vivo glomerular filtration function.
Garreta E, Prado P, Tarantino C, Oria R, Fanlo L, Martí E, et al. Fine tuning the extracellular environment accelerates the derivation of kidney organoids from human pluripotent stem cells. Nat Mater. 2019;18(4):397–405. Confirmed that implantation of kidney organoids in CAM promoted differentiation and growth, and soft hydrogel promotes expression of genes associated with mesoderm differentiation.
Bantounas I, Ranjzad P, Tengku F, Silajdžić E, Forster D, Asselin M-C, et al. Generation of functioning nephrons by implanting human pluripotent stem cell-derived kidney progenitors. Stem Cell Rep. 2018;10(3):766–779. Proved that implanted kidney progenitors can form organ-like masses, and glomerular-like structures in the implants possess characteristic arrangements of podocytes as observed in vivo.
Bantounas I, Silajdžić E, Woolf AS, Kimber SJ. Formation of mature nephrons by implantation of human pluripotent stem cell-derived progenitors into mice. In: Gnudi L, Long DA, editors. Diabetic nephropathy: methods and protocols. New York: Springer US; 2020. p. 309–22. Described a step by step protocol for getting more advanced kidney structures comprising vascularized glomeruli and tubular elements.
Kumar Gupta A, Sarkar P, Wertheim JA, Pan X, Carroll TJ, Oxburgh L. Asynchronous mixing of kidney progenitor cells potentiates nephrogenesis in organoids. Commun Biol. 2020;3(1):1–11. Demonstrated that differentiation in organoids is most efficient from heterochronous mixes and asynchronous mixing promoted nephrogenesis.
Assawachananont J, Mandai M, Okamoto S, Yamada C, Eiraku M, Yonemura S, et al. Transplantation of embryonic and induced pluripotent stem cell-derived 3D retinal sheets into retinal degenerative mice. Stem Cell Rep. 2014;2(5):662–74.
Shirai H, Mandai M, Matsushita K, Kuwahara A, Yonemura S, Nakano T, et al. Transplantation of human embryonic stem cell-derived retinal tissue in two primate models of retinal degeneration. Proc Natl Acad Sci. 2016;113(1):E81–90.
Mandai M, Fujii M, Hashiguchi T, Sunagawa GA, Ito S-I, Sun J, et al. iPSC-derived retina transplants improve vision in rd1 end-stage retinal-degeneration mice. Stem Cell Rep. 2017;8(1):69–83.
Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5+ stem cell. Nat Med. 2012;18(4):618–23.
Fordham RP, Yui S, Hannan NR, Soendergaard C, Madgwick A, Schweiger PJ, et al. Transplantation of expanded fetal intestinal progenitors contributes to colon regeneration after injury. Cell Stem Cell. 2013;13(6):734–44.
Lindström NO, De Sena Brandine G, Tran T, Ransick A, Suh G, Guo J, et al. Progressive recruitment of mesenchymal progenitors reveals a time-dependent process of cell fate acquisition in mouse and human nephrogenesis. Dev Cell. 2018;45(5):651-660.e4.
Wu J, Platero-Luengo A, Sakurai M, Sugawara A, Gil MA, Yamauchi T, et al. Interspecies chimerism with mammalian pluripotent stem cells. Cell. 2017;168(3):473–486. e15.
Miyoshi T, Hiratsuka K, Saiz EG, Morizane R. Kidney organoids in translational medicine: disease modeling and regenerative medicine. Dev Dyn. 2020;249(1):34–45.
Golestaneh L, Alvarez PJ, Reaven NL, Funk SE, McGaughey KJ, Romero A, et al. All-cause costs increase exponentially with increased chronic kidney disease stage. Am J Manag Care. 2017;23(10 Suppl):S163–72.
Acknowledgements
The authors thank all members of Musah lab at Duke and Dr Minerva Matos-Garner from the Duke Graduate Communications and Intercultural Programs for comments on the manuscript.
Funding
This work was supported by a Genentech Research Award and a Whitehead Scholarship for Biomedical Science awarded to S.M. A.D.B. is grateful for a Duke-Alfred P. Sloan Foundation scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cellular Transplants
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kalejaiye, T.D., Barreto, A.D. & Musah, S. Translating Organoids into Artificial Kidneys. Curr Transpl Rep 9, 276–286 (2022). https://doi.org/10.1007/s40472-022-00383-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40472-022-00383-0