Abstract
Purpose
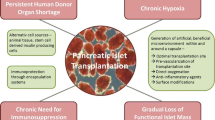
While exogenous insulin administration remains the cornerstone of management for type I diabetes mellitus (T1DM), patients now have a variety of options that include whole organ pancreas transplant, islet cell transplant, extracorporeal mechanical (bihormonal artificial pancreas), and implanted bioartificial (encapsulated islet) delivery systems. The purpose of this review is to compare these options and consider future developments.
Recent Results
Use of whole organ pancreas transplant has declined in the USA over the past several years. Pancreas transplant offers the most durable form of beta cell replacement, especially in the context of a simultaneous kidney-pancreas transplant or a pancreas after kidney transplant. However, pancreatic transplant is associated with significant perioperative morbidity including the risk of a leak of exocrine secretions. Despite a decline in popularity, pancreas transplant outcomes remain excellent. Pancreatic islet transplant offers the opportunity for beta cell replacement with less morbidity. Islet cell transplant outcomes have improved requiring fewer donor pancreata to achieve insulin independence. However, islet cell recipients continue to incur the risk of lifelong immunosuppression, and, to date, long-term islet cell transplant outcomes are not equal to whole organ transplant. Mechanical solutions have progressed significantly, as technology as enable continuous glucose monitoring and bihormonal (insulin and glucagon) pumps. This technology is limited by the sensitivity of the glucose monitor and the time to effective absorption and distribution of subcutaneous administration of hormones. Finally, bioartificial encapsulated islets are in early clinical trials. Encapsulation results in an immune privileged environment eliminating the need for immunosuppression. However, long-term survival of the islets in vivo has not been established, and other barriers (e.g., fibrin deposition occluding the pores of the encapsulated islet) remain to be overcome.
Summary
The optimal therapeutic choice for patients with type 1 diabetes must be personalized for the patient and address their specific comorbid conditions (specifically concomitant renal failure), surgical risk, and medical literacy. Increase options promise the opportunity for better glucose control and further reduction in secondary diabetic complications.
Similar content being viewed by others
Abbreviations
- T1DM:
-
Type I diabetes mellitus
- T2DM :
-
Type 2 diabetes mellitus
- PTA:
-
Pancreas transplanted alone
- AI:
-
Artificial intelligence
- ESKD:
-
End stage kidney disease
- PAK:
-
Pancreas transplant
- SPK:
-
Simultaneous kidney and pancreas transplantation
- MDI:
-
Multiple daily insulin injections
- ESC:
-
Embryonic stem cells
- iPSC:
-
Induced pluripotent stem cells
- APDS:
-
Artificial pancreas device system
- CGM:
-
Continuous glucose monitoring system
- SMBG:
-
Self-monitoring of glucose
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Heinemann L, Freckmann G, Ehrmann D, Faber-Heinemann G, Guerra S, Waldenmaier D, et al. Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet. 2018;391(10128):1367–77.
Perlmuter LC, Flanagan BP, Shah PH, Singh SP. Glycemic control and hypoglycemia: is the loser the winner? Diabetes Care. 2008;31(10):2072–6.
Mian Z, Hermayer KL, Jenkins A. Continuous glucose monitoring: review of an innovation in diabetes management. Am J Med Sci. 2019;358(5):332–9.
Nath DS, Gruessner AC, Kandaswamy R, Gruessner RW, Sutherland DER, Humar A. Outcomes of pancreas transplants for patients with type 2 diabetes mellitus. Clin Transpl. 2005;19(6):792–7.
•• Scalea JR, et al. Pancreas transplantation in older patients is safe, but patient selection is paramount. Transpl Int. 2016;29(7):810–8. This was a single-center, retrospective study of various solid organ pancreas transplants and the survival benefits regardless of older age.
Montagud-Marrahi E, et al. Outcomes of pancreas transplantation in older diabetic patients. BMJ Open Diabetes Res Care. 2020;8(1).
Chandie Shaw PK, Vandenbroucke JP, Tjandra YI, Rosendaal FR, Rosman JB, Geerlings W, et al. Increased end-stage diabetic nephropathy in Indo-Asian immigrants living in the Netherlands. Diabetologia. 2002;45(3):337–41.
Maffi P, Secchi A. Islet transplantation alone versus solitary pancreas transplantation: an outcome-driven choice? Curr Diab Rep. 2019;19(5):26.
• Fiorina P, et al. Reversal of left ventricular diastolic dysfunction after kidney-pancreas transplantation in type 1 diabetic uremic patients. Diabetes Care. 2000;23(12):1804–10. This study evaluated the effects of glycometabolic control achieved by pancreas transplant on left ventricular function in uremic T1DM.
La Rocca E, et al. Patient survival and cardiovascular events after kidney-pancreas transplantation: comparison with kidney transplantation alone in uremic IDDM patients. Cell Transplant. 2000;9(6):929–32.
Giannarelli R, Coppelli A, Sartini MS, del Chiaro M, Vistoli F, Rizzo G, et al. Pancreas transplant alone has beneficial effects on retinopathy in type 1 diabetic patients. Diabetologia. 2006;49(12):2977–82.
Wang Q, Klein R, Moss SE, Klein BEK, Hoyer C, Burke K, et al. The influence of combined kidney-pancreas transplantation on the progression of diabetic retinopathy. A case series. Ophthalmology. 1994;101(6):1071–6.
Mittal S, Johnson P, Friend P. Pancreas transplantation: solid organ and islet. Cold Spring Harbor Perspec Med. 2014;4(4).
Lee TC, Barshes NR, O’Mahony CA, Nguyen L, Brunicardi FC, Ricordi C, et al. The effect of pancreatic islet transplantation on progression of diabetic retinopathy and neuropathy. Transplant Proc. 2005;37(5):2263–5.
Smith GC, Trauer T, Kerr PG, Chadban SJ. Prospective quality-of-life monitoring of simultaneous pancreas and kidney transplant recipients using the 36-item short form health survey. Am J Kidney Dis. 2010;55(4):698–707.
Ziaja J, Bożek-Pająk D, Kowalik A, Król R, Cierpka L. Impact of pancreas transplantation on the quality of life of diabetic renal transplant recipients. Transplant Proc. 2009;41(8):3156–8.
•• Shapiro AM, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318–30. This was a multisite international trial of Edmonton protocol for islet tansplantation that demonstrated successful longterm endogenous insulin production and glycemic stability in T1DM.
Shapiro AM, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343(4):230–8.
Hering BJ, Kandaswamy R, Ansite JD, Eckman PM, Nakano M, Sawada T, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA. 2005;293(7):830–5.
Drachenberg CB, Klassen DK, Weir MR, Wiland A, Fink JC, Bartlett ST, et al. Islet cell damage associated with tacrolimus and cyclosporine: morphological features in pancreas allograft biopsies and clinical correlation. Transplantation. 1999;68(3):396–402.
Campbell PM, Senior PA, Salam A, LaBranche K, Bigam DL, Kneteman NM, et al. High risk of sensitization after failed islet transplantation. Am J Transplant. 2007;7(10):2311–7.
•• Sakata N, et al. Encapsulated islets transplantation: past, present and future. World J Gastrointest Pathophys. 2012;3(1):19–26. This a thorough review of islet encapsulation strategy using human stem cells or xenogenic islets with immune-isolation providing biomembranes.
Pepper AR, Bruni A, Shapiro AMJ. Clinical islet transplantation: is the future finally now? Curr Opin Organ Transplant. 2018;23(4):428–39.
Buder B, Alexander M, Krishnan R, Chapman DW, Lakey JRT. Encapsulated islet transplantation: strategies and clinical trials. Immune Netw. 2013;13(6):235–9.
Matsumoto S, Noguchi H, Naziruddin B, Onaca N, Jackson A, Hatanaka N, et al. Improvement of pancreatic islet cell isolation for transplantation. Proc (Baylor Univ Med Cent). 2007;20(4):357–62.
Ozmen L, Ekdahl KN, Elgue G, Larsson R, Korsgren O, Nilsson B. Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets - possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes. 2002;51(6):1779–84.
Pagliuca FW, Millman JR, Gürtler M, Segel M, van Dervort A, Ryu JH, et al. Generation of functional human pancreatic beta cells in vitro. Cell. 2014;159(2):428–39.
• Jeon K, et al. Differentiation and transplantation of functional pancreatic beta cells generated from induced pluripotent stem cells derived from a type 1 diabetes mouse model. Stem Cells Dev. 2012;21(14):2642–55. This was an animal model trial studying the potential application of islets differentiated from pluripotent stem cells in curing T1DM.
de Vos P, Marchetti P. Encapsulation of pancreatic islets for transplantation in diabetes: the untouchable islets. Trends Mol Med. 2002;8(8):363–6.
Calafiore R. Microencapsulation for cell therapy of type 1 diabetes mellitus: the interplay between common beliefs, prejudices and real progress. J Diab Investigat. 2018;9(2):231–3.
Pedraza E, Coronel MM, Fraker CA, Ricordi C, Stabler CL. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proc Natl Acad Sci U S A. 2012;109(11):4245–50.
Su J, Hu BH, Lowe WL Jr, Kaufman DB, Messersmith PB. Anti-inflammatory peptide-functionalized hydrogels for insulin-secreting cell encapsulation. Biomaterials. 2010;31(2):308–14.
Kizilel S, Scavone A, Liu X, Nothias JM, Ostrega D, Witkowski P, et al. Encapsulation of pancreatic islets within nano-thin functional polyethylene glycol coatings for enhanced insulin secretion. Tissue Eng Part A. 2010;16(7):2217–28.
Calafiore R, Basta G. Clinical application of microencapsulated islets: actual prospectives on progress and challenges. Adv Drug Deliv Rev. 2014;67-68:84–92.
Basta G, Montanucci P, Luca G, Boselli C, Noya G, Barbaro B, et al. Long-term metabolic and immunological follow-up of nonimmunosuppressed patients with type 1 diabetes treated with microencapsulated islet allografts. Diabetes Care. 2011;34(11):2406–9.
Ekser B, Li P, Cooper DKC. Xenotransplantation: past, present, and future. Curr Opin Organ Transplant. 2017;22(6):513–21.
Montanucci P, Alunno A, Basta G, Bistoni O, Pescara T, Caterbi S, et al. Restoration of t cell substes of patients with type 1 diabetes mellitus by microencapsulated human umbilical cord Wharton jelly-derived mesenchymal stem cells: an in vitro study. Clin Immunol. 2016;163:34–41.
Rezania A, Bruin JE, Riedel MJ, Mojibian M, Asadi A, Xu J, et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61(8):2016–29.
Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–52.
Russell SJ, el-Khatib FH, Sinha M, Magyar KL, McKeon K, Goergen LG, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med. 2014;371(4):313–25.
• El-Khatib FH, Balliro C, Hillard MA. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial (vol 389, pg 369, 2016). Lancet. 2017;389(10067):368–8. This was a comparison study between autonomous adaptive dosing algorithms between a bihormonal (insilin+glucagon) and conventional insulin pump therapy to achieve glycemic regulation.
Thabit H, Tauschmann M, Allen JM, Leelarathna L, Hartnell S, Wilinska ME, et al. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med. 2015;373(22):2129–40.
Garg SK, Weinzimer SA, Tamborlane WV, Buckingham BA, Bode BW, Bailey TS, et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther. 2017;19(3):155–63.
Christiansen SC, Fougner AL, Stavdahl Ø, Kölle K, Ellingsen R, Carlsen SM. A review of the current challenges associated with the development of an artificial pancreas by a double subcutaneous approach. Diabetes Ther. 2017;8(3):489–506.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pancreas Transplantation
Rights and permissions
About this article
Cite this article
Manay, P., Turgeon, N. & Axelrod, D.A. Role of Whole Organ Pancreas Transplantation in the Day of Bioartificial and Artificial Pancreas. Curr Transpl Rep 7, 223–229 (2020). https://doi.org/10.1007/s40472-020-00300-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40472-020-00300-3