Abstract
The aim of this systematic review and meta-analysis was to assess the diagnostic performance of 18F/11C-labeled choline positron emission tomography (PET) or PET/computed tomography (CT) in the detection of prostate cancer and its metastases in comparison with histology of primary prostate cancer, other tracers and other imaging modalities. A PubMed and Web of Knowledge search was carried out to select English language articles, published before October 2012, dealing with the diagnostic performance of 18F- and 11C-labeled choline PET in the detection of prostate cancer in both staging and restaging, comparing it with histology of primary tumor, other imaging modalities and other tracers. Articles were included only if absolute numbers of true positive, true negative, false positive and false negative test results were available or derivable from the text. Reviews, clinical reports, and editorial articles were excluded. We re-analyzed all complete studies, performing qualitative and quantitative analyses. For the period 2003 to October 2012, we found 40 complete articles that critically evaluated the role of radiolabeled choline PET in comparison with histology of primary prostate cancer, magnetic resonance imaging (MRI), 18F-fluorodeoxyglucose (18F-FDG) PET/CT or other imaging modalities in prostate cancer patients. A meta-analysis was carried out on eight selected studies, comprising a total of 276 analyzed patients. The meta-analysis gave a pooled sensitivity of 62.6 % (95 % CI: 54–70.6) vs. 59.7 % (95 % CI: 51.1–67.9), and a pooled specificity of 76.3 % (95 % CI: 65.4–85.1) vs 76.1 % (95 % CI: 65.9–84.6) for radiolabeled choline PET/CT as compared to MRI for the detection of the primary lesion; a pooled sensitivity of 72 % (95 % CI: 66.9–76.6), and a pooled specificity of 61.6 % (95 % CI: 55.1–67.7) for the identification of the primary tumor in comparison with step section histopathology, and finally a pooled sensitivity of 65.1 % (95 % CI: 53.8–75.2) for radiolabeled choline PET/CT vs 39.8 (95 % CI: 29.2–51.1) for 18F-FDG PET/CT in the detection of prostate cancer metastases. Heterogeneity ranged between 0.0 and 94.0 %. The use of radiolabeled choline PET for the detection of primary prostate cancer may be unnecessary. On the contrary, radiolabeled choline PET and PET/CT are useful techniques in the detection of loco-regional and distant metastases in prostate cancer patients, being more sensitive than other tracers and other imaging modalities in any given clinical scenario.
Similar content being viewed by others
Introduction
A substantial number of men receiving a prostate cancer (PCa) diagnosis will initially show advanced disease [1] or will experience cancer relapse after initial local treatment (radical prostatectomy—RP or external beam radiotherapy—EBRT). The accuracy of conventional imaging (CI) for PCa staging and restaging is poor. Both contrast-enhanced abdominopelvic computed tomography (CT) and whole-body bone scintigraphy (BS) are of limited utility in patients with low-risk primary disease or relapsed disease with only moderately elevated prostate-specific antigen (PSA) levels [2–4]. Although these imaging modalities are widely used in first-line imaging workup, American and European guidelines do not recommend them [5]. At initial staging, transrectal ultrasonography (TRUS) is usually the first imaging method used and its role, primarily, is to guide prostatic needle biopsy. However, in view of the increase in organ-sparing treatment options, precise evaluation of intraprostatic extension of PCa is gaining clinical importance. Magnetic resonance imaging (MRI) is widely employed to detect PCa lesions; however, detectability of this disease is limited, even when MRI is combined with MR spectroscopy (MRS) [6–9]. Furthermore, 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET) is of only minimal utility for the evaluation of organ-confined PCa [10, 11].
Restaging and follow-up of PCa is typically performed using whole-body BS, the most sensitive test for detecting skeletal metastasis, and abdominopelvic contrast CT for detection of soft tissue malignancy, predominantly pelvic nodal involvement. These and other imaging modalities including 18F-FDG PET have shown limited accuracy [11–14].
Many recently published reports have highlighted the potential advantages offered by PET with new radiotracers such as 18F/11C-labeled choline and 11C-acetate in the assessment of PCa patients [15–18]. The utility of these tracers is based on the increased cell proliferation in tumors and the up-regulation of choline kinase in cancer cells [14].
This systematic review and meta-analysis sets out to provide an overview of the diagnostic performance, in PCa, of 18F/11C-labeled choline PET or PET/CT in the detection of primary tumor and loco-regional and distant metastases, in comparison with histology of primary PCa, other tracers and other imaging modalities.
Materials and methods
Literature search
A computer search of the literature was performed to identify studies, in humans, that compared the diagnostic performance of radiolabeled choline PET or radiolabeled choline PET/CT with other imaging modalities or tracers used for the detection of PCa. We also included studies validating the results of radiolabeled choline PET with histology of primary PCa alone. The PubMed and ISI Web of Knowledge databases from 2000 to October 2012 were searched using the following key words “prostatic neoplasm” or “prostatic” and “neoplasm” or “prostate” and “cancer” or “prostate cancer” and “choline pet” or “prostate cancer” and “choline PET/CT”. For the Medline search, the following limits were used: species (human), article type (reviews and systematic reviews, clinical trials and randomized clinical trials, original articles, comparative studies, and multicenter studies), and language (English). The references of articles found in the literature search were also examined to identify additional reports that met the inclusion criteria. In each of these series, we looked for the following items: number of patients, mean or median age, design of the study, reference standard, sensitivity, specificity, and other diagnostic data from radiolabeled choline PET or PET/CT scans. Articles giving results of 18F/11C-labeled choline PET or PET/CT studies and containing information on primary PCa histological specimens, other imaging modalities (MRI or CT or BS) or other tracers in PCa patients and published in English were reviewed. The list of articles was supplemented through extensive cross-checking of the reference lists of all the retrieved articles.
Selection of studies
Two experienced nuclear medicine physicians (LE and ARC) independently checked the retrieved articles. Disagreements were resolved by consensus. For the qualitative analysis, reports that included data on comparisons between histology of primary tumor, radiolabeled choline PET and other imaging modalities were included if: (a) the reference standard was pathology or other common imaging modalities, and (b) the sample size was ≥10 patients. Moreover, for the meta-analysis, articles were included if the absolute numbers of true positive (TP), false negative (FN), false positive (FP), and true negative (TN) test results were available or derivable from the articles, thereby allowing us to construct 2 × 2 contingency tables. Abstracts were excluded from this analysis on the grounds that they do not provide sufficient data to evaluate methodological quality and to allow calculation of diagnostic accuracy. Reviews, clinical reports and editorial comments were also excluded.
Data extraction
Three observers (LE, ARC, and MB), using a standardized form, independently extracted relevant data on study characteristics and examination results. The observers were not blinded to the information such as the journal name, authors’ names, authors’ affiliations, and year of publication, since this has been shown to be unnecessary [19]. The reviewers (ARC and MB) evaluated relevant studies using the Quality Assessment Tool for Diagnostic Accuracy Studies (QUADAS) criteria [20]. The evaluation was based on a 14-point scale. Each item was rated “yes,” “no,” or “unclear”. Inconsistent findings between the two readers were discussed and agreed upon by consensus (LE). For each included study, information was collected concerning the basic study (author name, journal, year of publication, country of origin, and study design), patients’ demographic and clinical characteristics (mean age, number of patients), technical parameters (type of radiopharmaceutical injected), and radiolabeled choline PET or PET/CT evaluation (visual or semi-quantitative analysis by standardized uptake value—SUV).
Statistical analysis
The numbers of TP, TN, FP, and FN results were extracted or computed from each selected study based on the radiolabeled choline PET as the index test. The analysis of each report was carried out according to the site of disease, the type of comparative imaging and the tracer. For each invasive and non-invasive technique, pooled sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), likelihood ratio (LR), accuracy and diagnostic odds ratio (DOR) were calculated with 95 % confidence intervals (CIs). We also calculated summary receiver operating characteristic (sROC) curves and areas under the curve (AUCs). A random effects model was used. Between-study heterogeneity was assessed using the Chi-squared and I-squared tests. The Chi-squared test provided an estimate of the between-study variance, while the I-squared test measured the proportion of inconsistency in individual studies that cannot be explained by chance. In accordance with Higgins et al. [21], the values of 25, 50, and 75 % for heterogeneity (I-squared) were considered low, moderate and high, respectively. The AUC was calculated to measure the accuracy of radiolabeled choline PET/CT in diagnosis of PCa versus other imaging techniques or tracers. All statistical analyses were performed using Meta-Disc statistical software version 1.4 (Unit of Clinical Biostatistics, Ramòn y Cajal Hospital, Madrid, Spain [22]).
Results
Identification and characteristics of studies
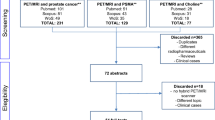
The search, using Mesh terms, of the PubMed and Web of Knowledge databases from 2000 to October 2012 generated 246 and 432 results, respectively. Thirty-seven and four original articles, respectively, were in English and dealt with comparative studies. Three articles were the same and were, therefore, considered only once. Six articles were identified through examination of the references of the retrieved studies. Twenty-three articles on the use, in PCa, of radiolabeled choline PET or PET/CT in comparison with other imaging techniques or other modalities were identified based on the inclusion criteria. Table 1 shows the characteristics of the identified studies. For the meta-analysis, we assessed the diagnostic performance of 18F/11C-labeled choline or PET/CT and other tools in eight original articles (Fig. 1, according to the PRISMA standard). Application of the QUADAS criteria showed these studies to be of a high quality (n = 8; score 11–14). The selected articles yielded data on a total of 276 patients (n = 89 for MRI, n = 89 for other tracers, and n = 98 for histology of primary PCa). Table 2 details the characteristics of the studies included in the meta-analysis. The entire population studied ranged in age from 41 to 88 years. Table 3 summarizes the diagnostic accuracy of each of the selected studies.
Radiolabeled choline PET vs. step section histopathology in primary tumor
Qualitative analysis
Kwee et al. [16], Farsad et al. [23], Martorana et al. [24], and Giovacchini et al. [25] compared the step-sectioned whole-mounted prostate specimen analysis with radiolabeled choline PET prior to RP. Kwee et al. [16] found a correlation between the maximum SUV (SUVmax) of malignant sextants and tumor size, assuming the SUV measured in a volume of tissue containing only malignant cells to be higher than that in a volume containing both malignant and benign cells. The authors, using an SUVmax threshold of 4.0, reported a sensitivity of 85 % and a specificity of 62 % for the detection of primary cancer. Farsad et al. [23], using six regions of interest (ROIs) and an SUVmax threshold of 2.5, found sensitivity, specificity, and accuracy values of 66, 81, and 71 %, respectively. Reske et al. [17] employing an SUVmax threshold of 2.65 obtained using 36 ROIs in prostate, reported a sensitivity of 81 %, a specificity of 87 %, and an accuracy of 84 %. Moreover, Scher et al. [26] performed a patient-based analysis of predominantly qualitative images analysis and provided a sensitivity of 86.5 %, a specificity of 61.9 %, and an accuracy of 77.6 % for radiolabeled choline PET and PET/CT in the detection of primary malignancy. Furthermore, these authors suggested that a cut-off value of 3.3, above which the SUVmax is considered to be malignant, would have yielded the best compromise between sensitivity and specificity, at 70.3 % and 57.1 %, respectively. Finally, in 2008, Giovacchini et al. [25] also argued that choline SUVmax had only moderate diagnostic accuracy in the assessment of histological findings.
Quantitative analysis (meta-analysis)
The sensitivity and specificity of radiolabeled choline PET/CT in the detection of primary PCa as compared to histology specimens ranged from 64 to 87 % and from 45.4 to 84.2 %, respectively, reaching a pooled sensitivity of 72 % (66.9–76.6 %) and a pooled specificity of 61.6 % (55.1–67.7 %). Likelihood ratio χ 2 test statistics were 19.49 (p < 0.001) and 36.70 (p < 0.001), respectively, for the sensitivity and specificity while the I 2 index values were 89.7 and 94.6 %, respectively; therefore, a high heterogeneity was shown. The SROC analysis reported an AUC value of 0.754 (p = 0.08).
Radiolabeled choline PET vs. MRI and TRUS in primary tumor
Qualitative analysis
Four reports from the literature compared radiolabeled choline PET and MRI in primary tumor detection [27–30], while one study focused on the difference in accuracy between MRI and radiolabeled choline PET/CT in the detection of local recurrence [31]. Rinnab et al. [32] compared the accuracy of 11C-labeled choline PET with TRUS, reporting that PET was more sensitive than TRUS for extracapsular extension, seminal vescicle involvement, and T4 tumors, but both methods understaged microscopic extension of the disease. Yamaguchi et al. [27] demonstrated the superiority of 11C-labeled choline PET with localization by SUVmax to predict laterality among primary lesions when compared with MRS using localization by the ratio of choline + creatinine to citrate (Cho + Cr/Ci ratio). These results led the authors to speculate that there is an essential difference in the significance of choline measurement between radiolabeled choline PET and MRS. Testa et al. [29] and Watanabe et al. [28] concluded that MRI had the highest sensitivity, while PET/CT showed good specificity; therefore, according to Van den Berg et al. [30], the association of MRI and SUVmax on radiolabeled choline PET/CT should be of benefit in primary tumor detection (sensitivity = 80.5 vs. 84.7 % for MRI alone and MRI plus SUVmax ≥2.7, respectively). On the other hand, Panebianco et al. [31] underlined the advantages of MRI over radiolabeled choline PET in the detection of local recurrence after surgical prostatectomy.
Quantitative analysis (meta-analysis)
Table 4 reports the results of the meta-analysis performed to compare MRI and radiolabeled choline PET or PET/CT in primary tumor detection. As illustrated, radiolabeled choline PET/CT showed a pooled sensitivity of 62.6 % (54.0–70.6 %) and a pooled specificity of 76.3 % (65.4–85.1 %), whereas MRI demonstrated a pooled sensitivity of 59.7 % (51.1–67.9 %) and a pooled specificity of 76.1 % (65.9–84.6 %). Heterogeneity was found both for MRI and radiolabeled choline PET studies, which was confirmed by the likelihood ratio χ 2 test and the I 2 index. There was no conclusive evidence of a cut-off effect for either radiolabeled choline PET or MRI according to Spearman correlation coefficients (ρ < 0.4), although an abnormal negative LR value was reported for radiolabeled choline PET (see Table 4). The AUCs were 0.743 (p = 0.24) and 0.737 (p = 0.05) for radiolabeled choline PET and MRI, respectively.
Radiolabeled choline PET vs. other tracers
Qualitative analysis
Langsteger et al. [33] and Beheshti et al. [34] studied 42 and 38 patients with PCa using both 18F-fluoride and 18F-labeled choline PET/CT for the detection of bone metastases. On lesion-based analysis, Beheshti et al. [34] reported sensitivity, specificity, and accuracy values of 74, 99 and 85 % for 18F-labeled choline PET/CT vs 81, 93, and 86 % for 18F-fluoride PET/CT, while Langsteger et al. [33], on site-based analysis, reported sensitivity, specificity, and accuracy values of 90, 96, and 95 % for 18F-labeled choline PET/CT and 87, 94, and 93 % for 18F-fluoride PET.
On the other hand, three articles compared the diagnostic performance of radiolabeled choline PET with that of FDG PET in detecting PCa recurrence [35–37]. In all cases, 18F-FDG did not seem indicated for the diagnosis of PCa, unless a poorly differentiated Gleason score group is considered. The different behaviors of the tracers indicate that choline is more sensitive in the detection of PCa relapse, while 18F-FDG seems better able to discriminate the proliferative character of the process.
Watanabe et al. [28] compared the accuracy of radiolabeled choline PET/CT vs 18F-FDG PET/CT in primary PCa, reporting sensitivity, specificity, and accuracy values of 73, 59, and 67 % vs. 31, 88, and 53 %, respectively.
Quantitative analysis (meta-analysis)
The pooled diagnostic performance results of the radiolabeled choline PET and 18F-FDG PET for all sites of disease in only two included studies are presented in Table 5. In all sites of disease, the sensitivity and specificity of radiolabeled choline PET in PCa patients ranged from 60.6 to 91.7 % and from 75.0 to 100 %, reaching a pooled sensitivity of 65.1 % (53.8–75.2 %) and a pooled specificity of 83.3 % (35.9–99.6 %), while the sensitivity and specificity of 18F-FDG PET in PCa patients ranged from 31.0 to 91.7 % and from 75.0 to 100 %, reaching a pooled sensitivity of 39.8 % (29.2–51.1 %) and a pooled specificity of 83.3 % (35.9–99.6 %). There was heterogeneity in only few cases, confirmed by both the likelihood ratio χ2 test and the I 2 index. No SROC curve was computed for radiolabeled choline and 18F-FDG PET due to the small number of studies considered.
Radiolabeled choline PET vs bone scan
Qualitative analysis
Bone metastasis is a common complication of PCa; about 65–75 % of men with advanced PCa demonstrated bone localizations of the disease [38]. CT, MRI, and BS have been widely used in restaging of the disease, but have shown low sensitivity especially for the detection of bone metastases [39, 40]. However, BS, due to its wide availability, low cost and considerable associated clinical experience, remains the initial study and the standard for the detection of bone lesions. Luboldt et al. [41] demonstrated that diffusion-weighted imaging appeared just as effective as 11C-labeled choline PET/CT in the detection of bone metastases in patients with PCa. Fuccio et al. [42] and Picchio et al. [43] evaluated the clinical utility of 11C-labeled choline PET/CT in patients with bone metastases, in comparison with a single lesion on BS and with multiple lesions on BS, respectively. In both studies, 11C-labeled choline PET/CT showed better sensitivity than BS in the detection of bone lesions but as suggested by Beheshti et al. [44], the comparison of an advanced tomographic modality like PET/CT with the planar only anterior and posterior views of BS may not provide reliable data on the basis of which to reach a conclusion, in particular for the vertebral spine, pelvic skeleton, and ribs. Moreover, PET/CT is able to detect early marrow-based metastases that constitute an important data for therapy monitoring.
Discussion
In most of the cited reports [16, 17, 24, 25], the authors concluded that radiolabeled choline PET, being associated with low accuracy, should not be used as a first-line technique for the initial diagnosis of primary PCa. The present meta-analysis gave a pooled sensitivity, a pooled specificity, and a DOR of 72, 61.6, and 4.84 % for radiolabeled choline PET in comparison with histological specimens, supporting the conclusions of the above-mentioned researchers.
In accordance with Watanabe et al. [28], MRI is recommended as a primary tool in the diagnosis of PCa, while radiolabeled choline PET imaging may serve in the detection of distant metastases when suspected. Moreover, as suggested by Yamaguchi et al. [27], when PCa is highly suspected due to a high serum PSA level but the results of biopsy are negative, obtaining information regarding localization of the main primary lesion by 11C-labeled choline PET may increase the success rate of biopsy and avoid unnecessary repeated biopsies, in turn reducing the discomfort for patients.
In the literature, radiolabeled choline PET has been compared with other tracers, such as 18F-FDG and fluoride with different end points [33, 36, 37, 45]. Radiolabeled choline PET is a convenient and non-invasive one-step procedure for the staging and restaging of PCa disease because in the initial assessment of high-risk PCa patients it can allow the early detection of bone marrow metastases [45], while in the restaging setting, radiolabeled choline PET can provide more important prognostic and therapeutic information than 18F-FDG PET, especially when the Gleason score and PSA value are high (for Gleason scores of 8–10, the overall accuracy was 50 vs. 65 %, while for PSA values >10 ng/mL, the overall accuracy was 33 vs. 100 %, respectively, for 18F-FDG and radiolabeled choline PET [37]).
According to McCarthy et al. [46], PET/CT with radiolabeled choline is able to differentiate malignant nodal involvement from reactive change, but this latter point should be better defined, given that a lack of sensitivity was reported in the staging setting by Evangelista et al. [47]. The issue of possible FP findings is of critical importance because increased 18F/11C-labeled choline uptake is not specific for carcinogenesis. Histological signs of inflammation have been found in lymph nodes with pathological 18F/11C-labeled uptake that was erroneously attributed to recurrent disease [48, 49]. False positive findings may also occur in the prostatectomy bed, although FN results are the greatest concern in this anatomical district [35].
Radiolabeled choline PET/CT is able to identify multiple bone sites of relapse and in many cases to identify extra-osseous lesions, too, even in the presence of low PSA values [43]; it therefore, emerges as an important tool in the restaging of PCa patients, as suggested by Beheshti et al. [44]. Almost all sclerotic lesions with Hounsfield Unit levels >825 are fluorocholine-negative, as reported by the same group [45], but under hormone therapy this pattern raises the possibility that these lesions may no longer be viable. A recent report by Giovacchini et al. [50] reported that PET/CT with 11C-labeled choline is able to detect PCa recurrence in about 10 % of patients who had increasing PSA after RP and no evidence of disease on CI.
In the present study, the difference in diagnostic performance between 18F- and 11C-labeled choline PET in the different clinical scenarios was not assessed due to the low number of relevant reports. In our opinion, Carbon-11 may, in accordance with its physiological elimination, be more useful than 18F-labeled choline PET for the identification of primary PCa and recurrent disease in the prostatic fossae, as reported by Richter et al. [37]. Conversely, Fluorine-18 is more accurate than 11C-labeled choline PET for the identification of lymph node involvement, in accordance with its small bowel accumulation. Both Carbon-11 and Fluorine-18 can be used for the identification of bone metastases. Prospective comparative studies across the tracers should be conducted.
The present study presents some limitations. First, the small number of studies included in the meta-analysis and the heterogeneity between the studies may have influenced the strength of the pooled results in the head-to-head comparison, as also suggested by the wide 95 % CIs. Second, publication bias was not assessed due to the small number of included studies. Third, the inclusion of English only studies in the systematic review could be considered a limitation of the research strategy. Finally, we searched only two data sources for articles, namely PubMed and ISI Web of Knowledge, and the lack of consultation other databases (i.e., SCOPUS or EMBASE) represents a limitation of the study.
Implications for practice. Radiolabeled choline PET/CT does not seem to be indicated for the detection of primary PCa due to its moderate sensitivity and specificity, unless an unexplained increase in PSA is found without any clear evidence of the disease. The advantage of a single scan for the detection of all sites of disease (lymph node and distant metastases) at initial staging, especially in high-risk patients and at restaging, should be considered, particularly when bone lesions are suspected, and a systemic treatment or targeted therapy are planned.
Implications for research. In summary, (1) 11C-labeled choline PET can be useful for guiding a biopsy in cases of PSA increase and no evidence of primary PCa tumor on CI (TRUS or MRI); (2) the diagnostic accuracy of 11C-labeled choline PET/CT in PCa is more accurate than that of conventional BS and 18F-FDG PET or PET/CT, and (3) radiolabeled choline PET/CT may be a “one-stop diagnostic procedure” for PCa both at initial staging and at restaging. In our opinion, a well-structured randomized prospective clinical trial comparing the prognostic value and cost-effectiveness of radiolabeled choline PET/CT in comparison with other imaging modalities should be planned.
References
Williams SG, Millar JL, Dally MJ, Sia S, Miles W, Duchesne GM (2004) What defines intermediate-risk prostate cancer? Variability in published prognostic models. Int J Radiat Oncol Biol Phys 58:11–18
Oesterling JE, Martin SK, Bergstralh EJ, Lowe FC (1993) The use of prostate-specific antigen in staging patients with newly diagnosed prostate cancer. JAMA 269:57–60
Platt JF, Bree RL, Schwab RE (1987) The accuracy of CT in the staging of carcinoma of the prostate. AJR Am J Roentgenol 149:315–318
Engeler CE, Wasseman NF, Zhang G (1992) Preoperative assessment of prostatic carcinoma by computerized tomography. Weaknesses and new perspectives. Urology 40:346–350
Heidenreich AH, Bolla M, Joniau S, Mason MD, Matveev V, Mottet N et al. (2010) Guidelines on prostate cancer. Eur Urol. available from the web-site: http://www.uroweb.org/gls/pdf/Prostate%20Cancer%202010.pdf
Wefer AE, Hricak H, Vigneron DB, Coakley FV, Lu Y, Wefer J et al (2000) Sextant localization of prostate cancer: comparison of sextant biopsy, magnetic resonance imaging and magnetic resonance spectroscopic imaging with step section histology. J Urol 164:400–404
Coakley FV, Kurhanewicz J, Lu Y, Jones KD, Swanson MG, Chang SD et al (2002) Prostate cancer tumor volume: measurement with endorectal MR and MR spectroscopic imaging. Radiology 223:91–97
Yu KK, Hricak H, Alagappan R, Chernoff DM, Bacchetti P, Zaloudek CJ (1997) Detection of extracapsular extension of prostate carcinoma with endorectal and phased-array coil MR imaging: multivariate feature analysis. Radiology 202:697–702
Thornbury JR, Ornestein D, Choyke PL, Langlotz CP, Weinreb JC (2001) Prostate cancer: what is the future role for imaging? AJR Am J Roentgenol 176:17–21
Liu IJ, Zafar MB, Lai YH, Segall GM, Terris MK (2001) Fluorodeoxyglucose positron emission tomography studies in diagnosis and staging of clinically organ-confined prostate cancer. Urology 57:108–111
Hoh CK, Seltzer MA, Franklin J, deKernion JB, Phelps ME, Belldegrun A (1998) Positron emission tomography in urological oncology. J Urol 159:347–356
Effert PJ, Bares R, Handt S, Wolff JM, Büll U, Jakse G (1996) Metabolic imaging of untreated prostate cancer by positron emission tomography with 18fluorine-labeled deoxyglucose. J Urol 155:994–998
Shreve PD, Grossman HB, Gross MD, Wahl RL (1996) Metastatic prostate cancer: initial findings of PET with 2-deoxy-2-[F-18] fluoro-d-glucose. Radiology 199:751–756
Schöder H, Larson S (2004) Positron emission tomography for prostate, bladder, and renal cancer. Semin Nucl Med 34:274–292
Cimitan M, Bortolus R, Morassut S, Canzonieri V, Garbeglio A, Baresic T et al (2006) [(18)F]fluorocholine PET/CT imaging for the detection of recurrent prostate cancer at PSA relapse: experience in 100 consecutive patients. Eur J Nucl Med Mol Imaging 33:1387–1398
Kwee SA, Wei H, Sesterhenn I, Yun D, Coel MN (2006) Localization of primary prostate cancer with dual-phase 18F-fluorocholine PET. J Nucl Med 47:262–269
Reske SN, Blumstein NM, Neumaier B, Gottfried HW, Finsterbusch F, Kocot D et al (2006) Imaging prostate cancer with 11C-choline PET/CT. J Nucl Med 47:1249–1254
Seltzer MA, Jahan SA, Sparks R, Stout DB, Satyamurthy N, Dahlbom M et al (2004) Radiation dose estimates in humans for (11)C-acetate whole-body PET. J Nucl Med 45:1233–1236
Berlin JA (1997) Does blinding of readers affect the results of meta-analyses? University of Pennsylvania Meta-analysis Blinding Study Group. Lancet 350:185–186
Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J (2003) The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 3:25
Higgins JP, Thompson S, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A (2006) Mata-Disc: a software for meta-analysis of test accuracy data. BMC Med Res Methodolol 6:31
Farsad M, Schiavina R, Castellucci P, Nanni C, Corti B, Martorana G et al (2005) Detection and localization of prostate cancer: correlation of 11C-choline PET/CT with histopathologic step-section analysis. J Nucl Med 46:1642–1649
Martorana G, Schiavina R, Corti B, Farsad M, Salizzoni E, Brunocilla E et al (2006) 11C-choline positron emission tomography/computerized tomography for tumor localization of primary prostate cancer in comparison with 12-core biopsy. J of Urol 176:954–960
Giovacchini G, Picchio M, Coradeschi E, Scattoni V, Bettinardi V, Corazzini C et al (2008) [(11C)]choline uptake with PET/CT for the initial diagnosis of prostate cancer: relation to PSA levels, tumour stage and anti-androgenic therapy. Eur J Nucl Med Mol Imaging 35:1065–1073
Scher B, Seitz M, Albinger W, Tiling W, Tiling R, Scherr M et al (2007) Value of 11C-choline PET and PET/CT in patients with suspected prostate cancer. Eur J Nucl Med Mol Imaging 34:45–53
Yamaguchi T, Lee J, Uemura H, Sasaki T, Takahashi N, Oka T et al (2005) Prostate cancer: a comparative study of 11C-choline PET and MR imaging combined with proton MR spetroscopy. Eur J Nucl Med Mol Imaging 32:742–748
Watanabe H, Kanematsu M, Kondo H, Kako N, Yamamoto N, Yamada T et al (2010) Preoperative detection of prostate cancer: a comparison with 11C-choline PET, 18F-fluorodeoxyglucose PET and MR imaging. J Magn Reson Imaging 31:1151–1156
Testa C, Schiavina R, Lodi R, Salizzoni E, Corti B, Farsad M et al (2007) Prostate cancer: sextant localization with MRI imaging, MR spectroscopy, and 11C-choline PET/CT. Radiology 244:797–806
Van den Bergh L, Koole M, Isebaert S, Joniau S, Deroose CM, Oyen R et al (2012) Is there an additional value of 11C-choline PET-CT to T2-weighted MRI images in the localization of intraprostatic tumor nodules? Inter J Rad Oncol Biol Phys 83:1486–1492
Panebianco V, Sciarra A, Lisi D, Galati F, Buonocore V, Catalano C et al (2012) Prostate cancer: 1HMRS-DCEMR at 3 T versus (18F)choline PET/CT in the detection of local prostate cancer recurrence in men with biochemical progression after radical retropubic prostatcetomy (RRP). Eur J Radiol 81:700–708
Rinnab L, Blumstein NM, Mottaghy FM, Hautmann RE, Kufer R, Hohl K, Reske SN (2007) 11C-choline positron emission tomography/computed tomography and transurectal ultrasonography for staging localized prostate cancer. BJU Int 99:1421–1426
Langsteger W, Balogova S, Huchet V, Beheshti M, Paycha F, Egrot C et al (2011) Fluorocholine (18F) and sodium fluoride (18F) PET/CT in the detection of prostate cancer: prospective comparison of diagnostic performance determined by masked reading. QJNMMI 55:448–457
Beheshti M, Vali R, Waldenberger P, Fitz F, Nader M, Loidl W et al (2008) Detection of bone metastases in patients with prostate cancer by 18F fluorocholine and 18F fluoride PET-CT: a comparative study. Eur J Nucl Med Mol Imaging 35:1766–1774
Picchio M, Messa C, Landoni C, Gianolli L, Sironi S, Brioschi M et al (2003) Value of (11C) choline positron emission tomography for re-staging prostate cancer: a comparison with (18F)fluorodeoxyglucose-positron emission tomography. J Urol 169:1337–1340
Beauregard JM, Williams SG, DeGrado TR, Roselt P, Hicks RJ (2010) Pilot comparison of 18F-fluorocholine and 18F-fluorodeoxyglucose PET/CT with conventional imaging in prostate cancer. JMIRO 54:325–332
Richter JA, Rodriguez M, Rioja J, Panuelas I, Marti-Climent J, Garrastachu P et al (2010) Dual tracer 11C-choline and FDG-PET in the diagnosis of biochemical prostate cancer relapse after radical treatment. Mol Imaging Biol 12:210–217
Coleman RE (2001) Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 27:165–176
Krämer S, Görich J, Gottfried HW, Riska P, Aschoff AJ, Rilinger N et al (1997) Sensitivity of computed tomography in detecting local recurrence of prostatic carcinoma following radical prostatectomy. Br J Radiol 70:995–999
Bott SR (2004) Prostate cancer: management of recurrent disease after radical prostatectomy. Prostatic Dis 7:211–216
Luboldt W, Kufer R, Blumstein N, Toussaint TL, Kluge A, Seeman MD, Luboldt H (2008) Prostate carcinoma: diffusion-weighted imaging as potential alternative to conventional MR and 11C-choline PET/CT for detection of bone metastases. Radiology 249:1017–1025
Fuccio C, Castellucci P, Schiavina R, Santi I, Allegri V, Pettinato V et al (2010) Role of 11C-choline PET/CT in the restaging of prostate cancer patients showing a single lesion on bone scintigraphy. Ann Nucl Med 24:485–492
Picchio M, Spinapolice EG, Fallanca F, Crivellaro C, Giovacchini G, Gianolli L, Messa C (2012) (11C) choline PET/CT detection in bone metastases in patients with PSA progression after primary treatment for prostate cancer: comparison with bone scintigraphy. Eur J of Nucl Med Mol Imaging 39:13–26
Beheshti M, Langsteger W (2011) Choline PET/CT compared with bone scintigraphy in the detection of bone metastases in prostate cancer. Eur J Nucl Med Mol Imaging 39:910–911
Beheshti M, Vali R, Waldenberger P, Fitz F, Nader M, Hammer J et al (2009) The use of F-18 Choline PET in the assessment of bone metastases in prostate cancer: correlation with morphological changes on CT. Mol Imaging Biol 12:98–107
McCarthy M, Siew T, Campbell A, Lenzo N, Spry N, Vivian J, Morandeau L (2011) 18F-fluoromethylcholine (FCH) PET imaging in patients with castration-resistant prostate cancer: prospective comparison with standard imaging. Eur J Nucl Med Mol Imaging 38:14–22
Evangelista L, Gutilla A, Zattoni F, Muzzio PC, Zattoni F (2012) Utility of choline positron emission tomography/computed tomography for lymph node involvement identification in intermediate- to high-risk prostate cancer: a systematic literature review and meta-analysis. Eur J Urol. doi:10.1016/j.eururo.2012.09.039
Schiavina R, Scattoni V, Castellucci P, Picchio M, Corti B, Briganti A et al (2008) 11C-choline positron emission tomography/computerized tomography for preoperative lymph node staging in intermediate-risk and high-risk prostate cancer: comparison with clinical staging nomograms. Eur Urol 54:392–398
Beheshti M, Imamovic L, Broiger G, Vali R, Waldenberger P, Stolber F et al (2010) 18F choline PET/CT in the preoperative staging of prostate cancer in patients with intermediate or high risk of extracapsular disease: a prospective study of 130 patients. Radiology 254:925–933
Giovacchini G, Picchio M, Briganti A, Cozzarini C, Scattoni V, Salonia A et al (2010) (11C)choline positron emission tomography/computerized tomography to restage prostate cancer cases with biochemical failure after radical prostatectomy and no disease evidence on conventional imaging. J Urol 184:938–943
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Evangelista, L., Cervino, A.R., Burei, M. et al. Comparative studies of radiolabeled choline positron emission tomography, histology of primary tumor and other imaging modalities in prostate cancer: a systematic review and meta-analysis. Clin Transl Imaging 1, 99–109 (2013). https://doi.org/10.1007/s40336-013-0016-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-013-0016-0