Abstract
Background
Patients who have had an Achilles tendon (AT) rupture repaired are potentially at higher risk for re-rupture than those without previous rupture. Little attention has been given to the neuromechanical modulation of muscle–tendon interaction and muscle activation profiles during human dynamic movements after AT rupture repair.
Objective
The purpose of this study was to examine muscle–tendon behavior and muscle activation during bilateral hopping.
Methods
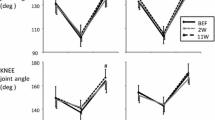
We enrolled nine subjects who had undergone surgical repair of unilateral AT rupture within the past 1–2 years. Subjects performed bilateral hopping while we took ultrasound, kinematic, and electromyogram recordings and measurements. AT behaviors were also recorded. We then compared responses between values obtained from the ruptured AT leg (LEGATR) and non-ruptured AT leg (LEGNOR).
Results
During hopping, the AT stretching amplitudes were greater in the LEGATR than in the LEGNOR, although the peak AT force and stiffness were smaller in the LEGATR than in the LEGNOR. The AT negative mechanical work did not show any significant differences between both legs. However, positive works were significantly lower in the LEGATR than in the LEGNOR. Electromyogram patterns in both soleus and tibialis anterior muscles clearly differed after ground contact for the LEGATR and the LEGNOR.
Conclusions
These results suggest that the repaired ruptured AT can be compliant and have insufficient Young’s modulus, which can influence mechanical responses in muscle activities. The modulation of agonist–antagonist muscle activities corresponding to the different levels of stiffness between the LEGATR and the LEGNOR may not be fully functioning during the pre-activation phase.







Similar content being viewed by others
References
Komi PV, Fukashiro S, Jarvinen M. Biomechanical loading of Achilles tendon during normal locomotion. Clin Sports Med. 1992;11:521–31.
Fukashiro S, Komi PV, Järvinen M, Miyashita M. In vivo Achilles tendon loading during jumping in humans. Eur J Appl Physiol Occup Physiol. 1995;71(5):453–8.
Wong J, Barrass V, Maffulli N. Quantitative review of operative and nonoperative management of Achilles tendon ruptures. Am J Sports Med. 2002;30(4):565–75.
Williams F, Mccullagh KG, Silver IA. The distribution of type I and III collagen and fibronectin in the healing equine tendon. Connect Tissue Res. 1984;12:211–27.
Hardy MA. The biology of scar formation. Phys Ther. 1989;69(12):1014–24.
Maffulli N, Ewen SW, Waterston SW, Reaper J, Barrass V. Tenocytes from ruptured and tendinopathic achilles tendons produce greater quantities of type III collagen than tenocytes from normal achilles tendons. An in vitro model of human tendon healing. Am J Sports Med. 2000;28(4):499–505.
Arya S, Kulig K. Tendinopathy alters mechanical and material properties of the Achilles tendon. J Appl Physiol. 2010;108(3):670–5.
Wyndow N, Cowan SM, Wrigley TV, Crossley KM. Triceps surae activation is altered in male runners with Achilles tendinopathy. J Electromyogr Kinesiol. 2013;23(1):166–72.
Wang HK, Chiang H, Chen WS, Shih TT, Huang YC, Jiang CC. Early neuromechanical outcomes of the triceps surae muscle-tendon after an Achilles’ tendon repair. Arch Phys Med Rehabil. 2013;94(8):1590–8.
Geremia JM, Bobbert MF, Casa Nova M, Ott RD, Lemos Fde A, Lupion Rde O, Frasson VB, Vaz MA. The structural and mechanical properties of the Achilles tendon 2 years after surgical repair. Clin Biomech. 2015;30(5):485–92.
Agres AN, Duda GN, Gehlen TJ, Arampatzis A, Taylor WR, Manegold S. Increased unilateral tendon stiffness and its effect on gait 2–6 years after Achilles tendon rupture. Scand J Med Sci Sports. 2015;25(6):860–7.
Baur H, Müller S, Hirschmüller A, Cassel M, Weber J, Mayer F. Comparison in lower leg neuromuscular activity between runners with unilateral mid-portion Achilles tendinopathy and healthy individuals. J Electromyogr Kinesiol. 2011;21(3):499–505.
Debenham JR, Travers MJ, Gibson W, Campbell A. Achilles tendinopathy alters stretch shortening cycle behaviour during a sub-maximal hopping task. J Sci Med Sport. 2016;19(1):69–73.
Fukutani A, Kurihara T. Comparison of the muscle fascicle length between resistance-trained and untrained individuals: cross-sectional observation. Springerplus. 2015;4:341. doi:10.1186/s40064-015-1133-1.
Stenroth L, Peltonen J, Cronin NJ, Sipilä S, Finni T. Age-related differences in Achilles tendon properties and triceps surae muscle architecture in vivo. J Appl Physiol. 2012;113(10):1537–44.
Hoffrén M, Ishikawa M, Avela J, Komi PV. Age-related fascicle-tendon interaction in repetitive hopping. Eur J Appl Physiol. 2012;112(12):4035–43.
Hoffrén-Mikkola M, Ishikawa M, Rantalainen T, Avela J, Komi PV. Neuromuscular mechanics and hopping training in elderly. Eur J Appl Physiol. 2015;115(5):863–77.
Stosic J, Finni T. Gastrocnemius tendon length and strain are different when assessed using straight or curved tendon model. Eur J Appl Physiol. 2011;111(12):3151–4.
Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol. 2000;10(5):361–74.
Grieve DW, Pheasant S, Cavanagh PR. Prediction of gastrocnemius length from knee and ankle joint posture. In: Asmussen E, Jorgensen K, editors. Biomechanics IV-A. Baltimore: University Park Press; 1978. p. 405–12.
Komi PV, Gollhofer A, Schmidtbleicher D, Frick U. Interaction between man and shoe in running: considerations for a more comprehensive measurement approach. Int J Sports Med. 1987;8(3):196–202.
Ishikawa M, Niemelä E, Komi PV. Interaction between fascicle and tendinous tissues in short-contact stretch-shortening cycle exercise with varying eccentric intensities. J Appl Physiol. 2005;99(1):217–23.
Arai A, Ishikawa M, Ito A. Agonist-antagonist muscle activation during drop jumps. Eur J Sport Sci. 2013;13(5):490–8.
Grey MJ, Ladouceur M, Andersen JB, Nielsen JB, Sinkjaer T. Group II muscle afferents probably contribute to the medium latency soleus stretch reflex during walking in humans. J Physiol. 2001;534(Pt 3):925–33.
Winter DA. Biomechanics and motor control of human movement. 2nd ed. New York: Wiley; 1990.
Rugg SG, Gregor RJ, Mandelbaum BR, Chiu L. In vivo moment arm calculations at the ankle using magnetic resonance imaging (MRI). J Biomech. 1990;23(5):495–501.
Kawakami Y, Muraoka T, Ito S, Kanehisa H, Fukunaga T. In vivo muscle fibre behaviour during counter-movement exercise in humans reveals a significant role for tendon elasticity. J Physiol. 2002;540(Pt 2):635–46.
Azevedo LB, Lambert MI, Vaughan CL, O’Connor CM, Schwellnus MP. Biomechanical variables associated with Achilles tendinopathy in runners. Br J Sports Med. 2009;43(4):288–92.
Chang YJ, Kulig K. The neuromechanical adaptations to Achilles tendinosis. J Physiol. 2015;593(15):3373–87.
Masood T, Kalliokoski K, Bojsen-Møller J, Magnusson SP, Finni T. Plantarflexor muscle function in healthy and chronic Achilles tendon pain subjects evaluated by the use of EMG and PET imaging. Clin Biomech. 2014;29(5):564–70.
Ishikawa M, Komi PV. Muscle fascicle and tendon behavior during human locomotion revisited. Exerc Sport Sci Rev. 2008;36(4):193–9.
Nicol C, Komi PV. Significance of passively induced stretch reflexes on Achilles tendon force enhancement. Muscle Nerve. 1998;21(11):1546–8.
Komi PV. Stretch-shortening cycle: a powerful model to study normal and fatigued muscle. J Biomech. 2000;33(10):1197–206.
Bohm S, Mersmann F, Marzilger R, Schroll A, Arampatzis A. Asymmetry of Achilles tendon mechanical and morphological properties between both legs. Scand J Med Sci Sports. 2015;25(1):e124–32.
Henriksen M, Aaboe J, Graven-Nielsen T, Bliddal H, Langberg H. Motor responses to experimental Achilles tendon pain. Br J Sports Med. 2011;45(5):393–8.
Finni T, Hodgson JA, Lai AM, Edgerton VR, Sinha S. Muscle synergism during isometric plantarflexion in achilles tendon rupture patients and in normal subjects revealed by velocity-encoded cine phase-contrast MRI. Clin Biomech (Bristol, Avon). 2006;21(1):67–74.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Hiroyuki Oda, Kanae Sano, Yoko Kunimasa, Paavo V. Komi, and Masaki Ishikawa have no conflicts of interest.
Funding
This work was supported by Japan Society for Promotion of Science (KAKENHI) Grant Numbers 26702026 and 15KK0261.
Rights and permissions
About this article
Cite this article
Oda, H., Sano, K., Kunimasa, Y. et al. Neuromechanical Modulation of the Achilles Tendon During Bilateral Hopping in Patients with Unilateral Achilles Tendon Rupture, Over 1 Year After Surgical Repair. Sports Med 47, 1221–1230 (2017). https://doi.org/10.1007/s40279-016-0629-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40279-016-0629-3