Abstract
Objective
This systematic literature review (SLR) had two objectives: to analyse published economic evaluations of biological disease-modifying anti-rheumatic drugs (bDMARDs) for patients with moderate to severe rheumatoid arthritis (RA) previously treated with DMARDs and to assess the quality of those that included sequences of treatments.
Methods
We performed an SLR on PubMed, Central, Cochrane, and French databases from January 2000 to December 2018. The search focused on cost-effectiveness/utility/benefit analyses. We extracted data on treatment sequences, outcomes (e.g. quality-adjusted life year) and choices of economic evaluation methods (e.g. model type, type of analysis, and method of utility estimation). We analysed the improvement of methods by comparing two sub-periods (2000–2009 and 2010–2018). The quality of reporting and the quality of the methods were assessed using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) and a set of eight key aspects for a reference case for economic evaluation of bDMARDs based on the Outcome Measures in Rheumatology (OMERACT) and Drummond checklists. Data extraction and study assessment were performed independently by two health economists.
Results
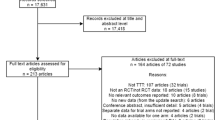
From the 824 records identified in the initial search, 51 publications were selected. Of these, 31 included sequences. Individual models such as discrete-event simulations were used in over two-fifths (22/51, 43%) of the selected studies. Few studies (7/51, 14%) used utility scores based on generic instruments (e.g. EQ-5D). Estimation of hospitalization costs was described in only approximately one-third of studies (19/51). Loss of quality of life (QoL) related to adverse events such as tuberculosis and pneumonia was included in one-tenth (5/51, 10%) of the studies. It was difficult to compare the results of the economic evaluations (i.e. incremental cost-effectiveness ratios) due to the high heterogeneity of studies in terms of disease stage, data sources, inputs, and methods of health outcome assessment used. For identified studies including sequences, the CHEERS assessment of reporting quality showed insufficient reporting of uncertainty analyses and utility weights in more than a third of the studies (11/31, 35%; 9/25, 36%). An in-depth assessment of the quality of the studies revealed that only seven, mostly conducted during the sub-period 2010–2018, addressed the majority of methodological quality assessment issues such as the simulation of patient sequence pathways, the use of systematic reviews and meta-analyses of comparative effectiveness, the choice of treatment sequence, and rules for switching.
Conclusion
Our SLR identified a lack of high-quality evaluations assessing bDMARD sequences, although some improvements were made in the reporting and modelling of patients’ pathways in studies published after 2010. In order to improve economic evaluations of RA, clear health technology assessment guidance on RA health-related QoL instruments must be provided, and data including long-term disease progression must be made available.

Similar content being viewed by others
Data Availability Statement
All valid data from the current review are presented in the electronic supplementary material.
References
Canadian Agency for Drugs and Technologies in Health. Drugs for the management of rheumatoid arthritis: clinical evaluation. Ottawa: CADTH; 2018. https://www.cadth.ca/sites/default/files/pdf/HT0010_RA_Report.pdf. Accessed July 25, 2019.
Gaujoux-Viala C, Gossec L, Cantagrel A, French Society for Rheumatology, et al. Recommendations of the French Society for Rheumatology for managing rheumatoid arthritis. Joint Bone Spine. 2014;81(4):287–97.
Simpson EL, Ren S, Hock ES, et al. Rheumatoid arthritis treated with 6-months of first-line biologic or biosimilar therapy: an updated systematic review and network meta-analysis. Int J Technol Assess Health Care. 2019;35(1):36–44.
Daien C, Hua C, Gaujoux-Viala C, et al. Update of French Society for Rheumatology recommendations for managing rheumatoid arthritis. Joint Bone Spine. 2019;86(2):135–50.
Zheng Y, Pan F, Sorensen S. Modeling treatment sequences in pharmacoeconomic models. Pharmacoeconomics. 2017;35(1):15–24.
Tosh J, Stevenson M, Akehurst R. Health economic modelling of treatment sequences for rheumatoid arthritis: a systematic review. Curr Rheumatol Rep. 2014;16(10):447.
Stevenson M, Archer R, Tosh J, Simpson E, Everson-Hock E, Stevens J, et al. Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for the treatment of rheumatoid arthritis not previously treated with disease-modifying antirheumatic drugs and after the failure of conventional disease-modifying antirheumatic drugs only: systematic review and economic evaluation. Health Technol Assess. 2016;20(35):1–610.
Institute for Clinical and Economic Review. Targeted immune modulators for rheumatoid arthritis: effectiveness and value. Draft evidence report. January 20, 2017. Boston. https://icer-review.org/wp-content/uploads/2016/08/NECEPAC_RA_Draft_Report_012017.pdf. Accessed July 25, 2019.
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
HAS. Evaluation médico-économique des traitements de fond biologiques. Annexes. https://www.has-sante.fr/portail/jcms/c_2580906/fr/evaluation-medico-economiquedes-traitements-de-fond-biologiques-dans-la-prise-en-charge-de-la-polyarthriterhumatoide. Accessed Nov 15, 2019.
Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ. 2013;346:f1049.
Gabriel SE, Drummond M, Maetzel A, Boers M, Coyle D, Welch V, et al. OMERACT 6 economics working group report: a proposal for a reference case for economic evaluation in rheumatoid arthritis. J Rheumatol. 2002;30(4):886–90.
Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Standard methods for the economic evaluation of health care programmes. Oxford: Oxford University Press; 2015.
Athanasakis K, Tarantilis F, Tsalapati K, Konstantopoulou T, Vritzali E, Kyriopoulos J. Cost-utility analysis of tocilizumab monotherapy in first line versus standard of care for the treatment of rheumatoid arthritis in Greece. Rheumatol Int. 2015;35(9):1489–95.
Beresniak A, Baerwald C, Zeidler H, Kruger K, Neubauer AS, Dupont D, et al. Cost-effectiveness simulation model of biologic strategies for treating to target rheumatoid arthritis in Germany. Clin Exp Rheumatol. 2013;31(3):400–8.
Brennan A, Bansback N, Reynolds A, Conway P. Modelling the cost-effectiveness of etanercept in adults with rheumatoid arthritis in the UK. Rheumatology. 2004;43(1):62–72.
Brennan A, Bansback N, Nixon R, Madan J, Harrison M, Watson K, et al. Modelling the cost effectiveness of TNF-alpha antagonists in the management of rheumatoid arthritis: results from the British Society for Rheumatology Biologics Registry. Rheumatology. 2007;46(8):1345–54.
Barbieri M, Wong JB, Drummond M. The cost effectiveness of infliximab for severe treatment-resistant rheumatoid arthritis in the UK. Pharmacoeconomics. 2005;23(6):607–18.
Bansback NJ, Brennan A, Ghatnekar O. Cost effectiveness of adalimumab in the treatment of patients with moderate to severe rheumatoid arthritis in Sweden. Ann Rheum Dis. 2005;64(7):995–1002.
Benucci M, Saviola G, Baiardi P, Manfredi M. Cost-effectiveness treatment with rituximab in patients with rheumatoid arthritis in real life. Rheumatol Int. 2011;31(11):1465–9.
Carlson JJ, Ogale S, Dejonckheere F, Sullivan SD. Economic evaluation of tocilizumab monotherapy compared to adalimumab monotherapy in the treatment of severe active rheumatoid arthritis. Value Health. 2015;18(2):173–9.
Diamantopoulos A, Finckh A, Huizinga T, Sungher DK, Sawyer L, Neto D, et al. Tocilizumab in the treatment of rheumatoid arthritis: a cost-effectiveness analysis in the UK. Pharmacoeconomics. 2014;32(8):775–87.
Eriksson JK, Karlsson JA, Bratt J, Petersson IF, van Vollenhoven RF, Ernestam S, et al. Cost-effectiveness of infliximab versus conventional combination treatment in methotrexate-refractory early rheumatoid arthritis: 2-year results of the register-enriched randomised controlled SWEFOT trial. Ann Rheum Dis. 2015;74(6):1094–101.
Hallinen TA, Soini EJ, Eklund K, Puolakka K. Cost-utility of different treatment strategies after the failure of tumour necrosis factor inhibitor in rheumatoid arthritis in the Finnish setting. Rheumatology. 2010;49(4):767–77.
Hidalgo-Vega A, Villoro R, Blasco JA, Talavera P, Ferro B, Purcaru O. Cost-utility analysis of certolizumab pegol versus alternative tumour necrosis factor inhibitors available for the treatment of moderate-to-severe active rheumatoid arthritis in Spain. Cost Eff Resour Alloc. 2015;13:11.
Kobelt G, Jönsson L, Young A, Eberhardt K. The cost-effectiveness of infliximab (Remicade) in the treatment of rheumatoid arthritis in Sweden and the United Kingdom based on the ATTRACT study. Rheumatology. 2003;42(2):326–35.
Kobelt G, Lindgren P, Singh A, Klareskog L. Cost effectiveness of etanercept (Enbrel) in combination with methotrexate in the treatment of active rheumatoid arthritis based on the TEMPO trial. Ann Rheum Dis. 2005;64(8):1174–9.
Kvamme MK, Lie E, Uhlig T, Moger TA, Kvien TK, Kristiansen IS. Cost-effectiveness of TNF inhibitors vs synthetic disease-modifying antirheumatic drugs in patients with rheumatoid arthritis: a Markov model study based on two longitudinal observational studies. Rheumatology. 2015;54(7):1226–35.
Lekander I, Borgstrom F, Svarvar P, Ljung T, Carli C, van Vollenhoven RF. Cost-effectiveness of real-world infliximab use in patients with rheumatoid arthritis in Sweden. Int J Technol Assess Health Care. 2010;26(1):54–61.
Lekander I, Kobelt G, Svarvar P, Ljung T, van Vollenhoven R, Borgstrom F. The comparison of trial data-based and registry data-based cost-effectiveness of infliximab treatment for rheumatoid arthritis in Sweden using a modeling approach. Value Health. 2013;16(2):251–8.
Lekander I, Borgstrom F, Lysholm J, van Vollenhoven RF, Lindblad S, Geborek P, et al. The cost-effectiveness of TNF-inhibitors for the treatment of rheumatoid arthritis in Swedish clinical practice. Eur J Health Econ. 2013;14(6):863–73.
Merkesdal S, Kirchhoff T, Wolka D, Ladinek G, Kielhorn A, Rubbert-Roth A. Cost-effectiveness analysis of rituximab treatment in patients in Germany with rheumatoid arthritis after etanercept-failure. Eur J Health Econ. 2010;11(1):95–104.
Nguyen CM, Bounthavong M, Mendes MA, Christopher ML, Tran JN, Kazerooni R, et al. Cost utility of tumour necrosis factor-alpha inhibitors for rheumatoid arthritis: an application of Bayesian methods for evidence synthesis in a Markov model. Pharmacoeconomics. 2012;30(7):575–93.
Puolakka K, Blafield H, Kauppi M, Luosujarvi R, Peltomaa R, Leikola-Pelho T, et al. Cost-effectiveness modelling of sequential biologic strategies for the treatment of moderate to severe rheumatoid arthritis in Finland. Open Rheumatol J. 2012;6(1):38–43.
Saraux A, Gossec L, Goupille P, Bregman B, Boccard E, Dupont D, et al. Cost-effectiveness modelling of biological treatment sequences in moderate to severe rheumatoid arthritis in France. Rheumatology. 2010;49(4):733–40.
Russell A, Beresniak A, Bessette L, Haraoui B, Rahman P, Thorne C, et al. Cost-effectiveness modeling of abatacept versus other biologic agents in DMARDS and anti-TNF inadequate responders for the management of moderate to severe rheumatoid arthritis. Clin Rheumatol. 2009;28(4):403–12.
Soini EJ, Hallinen TA, Puolakka K, Vihervaara V, Kauppi MJ. Cost-effectiveness of adalimumab, etanercept, and tocilizumab as first-line treatments for moderate-to-severe rheumatoid arthritis. J Med Econ. 2012;15(2):340–51.
Soini E, Asseburg C, Taiha M, Puolakka K, Purcaru O, Luosujarvi R. Modeled health economic impact of a hypothetical certolizumab pegol risk-sharing scheme for patients with moderate-to-severe rheumatoid arthritis in Finland. Adv Ther. 2017;34(10):2316–32.
Tanno M, Nakamura I, Ito K, Tanaka H, Ohta H, Kobayashi M, et al. Modeling and cost-effectiveness analysis of etanercept in adults with rheumatoid arthritis in Japan: a preliminary analysis. Mod Rheumatol. 2006;16(2):77–84.
Tanaka E, Inoue E, Hoshi D, Shimizu Y, Kobayashi A, Sugimoto N, et al. Cost-effectiveness of tocilizumab, a humanized anti-interleukin-6 receptor monoclonal antibody, versus methotrexate in patients with rheumatoid arthritis using real-world data from the IORRA observational cohort study. Mod Rheumatol. 2015;25(4):503–13.
Wu B, Wilson A, Wang FF, Wang SL, Wallace DJ, Weisman MH, et al. Cost effectiveness of different treatment strategies in the treatment of patients with moderate to severe rheumatoid arthritis in china. PLoS One. 2012;7(10):e47373.
Hashemi-Meshkini A, Nikfar S, Glaser E, Jamshidi A, Hosseini SA. Cost-effectiveness analysis of tocilizumab in comparison with infliximab in Iranian rheumatoid arthritis patients with inadequate response to tDMARDs: a multistage Markov model. Value Health Reg Issues. 2016;9:42–8.
Alemao E, Johal S, Al MJ, Rutten-van Molken M. Cost-effectiveness analysis of abatacept compared with adalimumab on background methotrexate in biologic-naive adult patients with rheumatoid arthritis and poor prognosis. Value Health. 2018;21(2):193–202.
Park SK, Park SH, Lee MY, Park JH, Jeong JH, Lee EK. Cost-effectiveness analysis of treatment sequence initiating with etanercept compared with leflunomide in rheumatoid arthritis: impact of reduced etanercept cost with patent expiration in South Korea. Clin Ther. 2016;38(11):2430–2446.e3.
Jalal H, O’Dell JR, Bridges SL Jr, Cofield S, Curtis JR, Mikuls TR, et al. Cost-effectiveness of triple therapy versus etanercept plus methotrexate in early aggressive rheumatoid arthritis. Arthritis Care Res. 2016;68(12):1751–7.
Cardenas M, de la Fuente S, Font P, Castro-Villegas M, Romero-Gomez M, Ruiz-Vilchez D, et al. Real-world cost-effectiveness of infliximab, etanercept and adalimumab in rheumatoid arthritis patients: results of the CREATE registry. Rheumatol Int. 2016;36(2):231–41.
Tzanetakos C, Tzioufas A, Goules A, Kourlaba G, Theodoratou T, Christou P, et al. Cost-utility analysis of certolizumab pegol in combination with methotrexate in patients with moderate-to-severe active rheumatoid arthritis in Greece. Rheumatol Int. 2017;37(9):1441–52.
Bansback N, Phibbs CS, Sun H, O’Dell JR, Brophy M, Keystone EC, et al. Triple therapy versus biologic therapy for active rheumatoid arthritis: a cost-effectiveness analysis. Ann Intern Med. 2017;167(1):8–16.
Jansen JP, Incerti D, Mutebi A, Peneva D, MacEwan JP, Stolshek B, et al. Cost-effectiveness of sequenced treatment of rheumatoid arthritis with targeted immune modulators. J Med Econ. 2017;20(7):703–14.
Beresniak A, Ariza-Ariza R, Garcia-Llorente JF, Ramirez-Arellano A, Dupont D. Modelling cost-effectiveness of biologic treatments based on disease activity scores for the management of rheumatoid arthritis in Spain. Int J Inflamm. 2011;2011:727634.
Cimmino MA, Leardini G, Salaffi F, Intorcia M, Bellatreccia A, Dupont D, et al. Assessing the cost-effectiveness of biologic agents for the management of moderate-to-severe rheumatoid arthritis in anti-TNF inadequate responders in Italy: a modelling approach. Clin Exp Rheumatol. 2011;29(4):633–41.
Welsing PM, Severens JL, Hartman M, van Riel PL, Laan RF. Modeling the 5-year cost effectiveness of treatment strategies including tumor necrosis factor-blocking agents and leflunomide for treating rheumatoid arthritis in the Netherlands. Arthritis Rheumatol. 2004;51(6):964–73.
Yuan Y, Trivedi D, Maclean R, Rosenblatt L. Indirect cost-effectiveness analyses of abatacept and rituximab in patients with moderate-to-severe rheumatoid arthritis in the United States. J Med Econ. 2010;13(1):33–41.
Vera-Llonch M, Massarotti E, Wolfe F, Shadick N, Westhovens R, Sofrygin O, et al. Cost-effectiveness of abatacept in patients with moderately to severely active rheumatoid arthritis and inadequate response to tumor necrosis factor-alpha antagonists. J Rheumatol. 2008;35(9):1745–53.
Joensuu JT, Aaltonen KJ, Aronen P, Sokka T, Puolakka K, Tuompo R, et al. Cost-effectiveness of biologic compared with conventional synthetic disease-modifying anti-rheumatic drugs in patients with rheumatoid arthritis: a Register study. Rheumatology. 2016;55(10):1803–11.
Chiou CF, Choi J, Reyes CM. Cost-effectiveness analysis of biological treatments for rheumatoid arthritis. Expert Rev Pharmacoeconomics Outcomes Res. 2004;4(3):307–15.
Marra CA, Marion SA, Guh DP, Najafzadeh M, Wolfe F, Esdaile JM, et al. Not all “quality-adjusted life years” are equal. J Clin Epidemiol. 2007;60(6):616–24.
Claxton L, Taylor M, Gerber RA, Gruben D, Moynagh D, Singh A, et al. Modelling the cost-effectiveness of tofacitinib for the treatment of rheumatoid arthritis in the United States. Curr Med Res Opin. 2018;34(11):1991–2000.
Chen YF, Jobanputra P, Barton P, Jowett S, Bryan S, Clark W, et al. A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness. Health Technol Assess. 2006;10(42):1–229.
Barton P, Jobanputra P, Wilson J, Bryan S, Burls A. The use of modelling to evaluate new drugs for patients with a chronic condition: the case of antibodies against tumour necrosis factor in rheumatoid arthritis. Health Technol Assess. 2004;8(11):1–110.
Jobanputra P, Barton P, Bryan S, Burls A. The effectiveness of infliximab and etanercept for the treatment of rheumatoid arthritis: a systematic review and economic evaluation. Health Technol Assess. 2002;6(21):1–110.
Malottki K, Barton P, Tsourapas A, Uthman AO, Liu Z, Routh K, et al. Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure of a tumour necrosis factor inhibitor: a systematic review and economic evaluation. Health Technol Assess. 2011;15(14):1–278.
Hernández Alava M, Wailoo A, Wolfe F, Michaud K. The relationship between EQ-5D, HAQ and pain in patients with rheumatoid arthritis. Rheumatology (Oxford). 2013;52(5):944–50.
Wolfe F, Michaud K, Gefeller O, Choi HK. Predicting mortality in patients with rheumatoid arthritis. Arthritis Rheumatol. 2003;48(6):1530–42.
Michaud K, Vera-Llonch M, Oster G. Mortality risk by functional status and health-related quality of life in patients with rheumatoid arthritis. J Rheumatol. 2012;39(1):54–9.
Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB, ISPOR-SMDM Modeling Good Research Practices Task Force. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Med Decis Making. 2012;32(5):733–43.
McCabe C, Dixon S. Testing the validity of cost-effectiveness models. Pharmacoeconomics. 2000;17(5):501–13.
de La Forest Divonne M, Gottenberg JE, Salliot C. Revue systématique des registres de polyarthrites rhumatoïdes sous biothérapie dans le monde et méta-analyse sur les données de tolérance. Revue du Rhumatisme. 2017;84(3):199–207.
Scholz S, Mittendorf T. Modeling rheumatoid arthritis using different techniques: a review of model construction and results. Health Econ Rev. 2014;4(1):18.
Sullivan SD, Alfonso-Cristancho R, Carlson J, Mallya U, Ringold S. Economic consequences of sequencing biologics in rheumatoid arthritis: a systematic review. J Med Econ. 2013;16(3):391–6.
Heather EM, Payne K, Harrison M, Symmons DP. Including adverse drug events in economic evaluations of anti-tumour necrosis factor-alpha drugs for adult rheumatoid arthritis: a systematic review of economic decision analytic models. Pharmacoeconomics. 2014;32(2):109–34.
Alemao E, Al MJ, Boonen AA, Stevenson MD, Verstappen SMM, Michaud K, Weinblatt ME, Rutten-van Mölken MPMH. Conceptual model for the health technology assessment of current and novel interventions in rheumatoid arthritis. PLoS One. 2018;13(10):e0205013.
Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int J Technol Assess Health Care. 2005;21(2):240–5.
Darkwah CC, van Gils PF, Hiligsmann M, Evers SM. Risk of bias in model-based economic evaluations: the ECOBIAS checklist. Expert Rev Pharmacoecon Outcomes Res. 2016;16(4):513–23.
Uttley L, Bermejo I, Ren S, Martyn-St James M, Wong R, Scott DL, et al. Tofacitinib for treating rheumatoid arthritis after the failure of disease-modifying anti-rheumatic drugs: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2018;36(9):1063–72.
Ren S, Bermejo I, Simpson E, Wong R, Scott DL, Young A, et al. Baricitinib for previously treated moderate or severe rheumatoid arthritis: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2018;36(7):769–78.
Bermejo I, Ren S, Simpson E, Clowes M, Scott DL, Young A, et al. Sarilumab for previously-treated moderate or severe rheumatoid arthritis: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics. 2018;36(12):1427–37.
Ghabri S, Stevenson M, Möller J, Caro JJ. Trusting the results of model-based economic analyses: is there a pragmatic validation solution? Pharmacoeconomics. 2019;37(1):1–6.
Acknowledgements
The authors are particularly grateful to Jaime Caro for his valuable comments on previous drafts of the manuscript. The authors are grateful to the following people: the rheumatoid arthritis working group (Morgane Beck, Aymeric Binard, Yves-Marie Pers, Franck Maunoury, and Sandrine Rollot) for their comments on the protocol for the systematic literature review of economic evaluations of biological treatment sequences; Catherine Le Galès Lionel Perrier, Sophie Cote, and Dominic Thorrington for their helpful comments and suggestions; and Gaëlle Fanelli and Yasmine Lombry for their help in the literature search. The authors would like to thank the anonymous reviewers and the Editor in Chief for their careful reading of the manuscript and insightful comments and suggestions.
Author information
Authors and Affiliations
Contributions
The authors contributed to the article in the following ways: SG conceptualized and wrote the manuscript, which was critically reviewed by LL, FB, and H-MS. LL participated in the process of validating the checklists. All authors participated in the research and approved the finalized version of the manuscript. SG acts as the overall guarantor for the manuscript content.
Corresponding author
Ethics declarations
Funding
The study was supported by the French National Authority for Health (Haute Autorité de la Santé, HAS).
Conflict of interest
The authors have no conflict of interest to declare. Salah Ghabri is employed by HAS. Laurent Lam is employed by AP-HP (Assistance Publique–Hôpitaux de Paris). Hans-Martin Spath is employed by the University of Claude Bernard Lyon 1. François Bocquet is employed by the University of Nantes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghabri, S., Lam, L., Bocquet, F. et al. Systematic Literature Review of Economic Evaluations of Biological Treatment Sequences for Patients with Moderate to Severe Rheumatoid Arthritis Previously Treated with Disease-Modifying Anti-rheumatic Drugs. PharmacoEconomics 38, 459–471 (2020). https://doi.org/10.1007/s40273-020-00887-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-020-00887-6