Abstract
Introduction
Expert judgement has a role in model-based economic evaluations (EEs) of healthcare interventions. This study aimed to produce reporting criteria for two types of study design to use expert judgement in model-based EE: (i) an expert elicitation (quantitative) study; and (ii) a Delphi study to collate (qualitative) expert opinion.
Methods
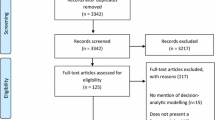
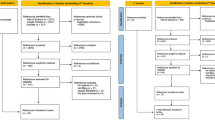
A two-round online Delphi process identified the degree of consensus for four core definitions (expert; expert parameter values; expert elicitation study; expert opinion) and two sets of reporting criteria in a purposive sample of experts. The initial set of reporting criteria comprised 17 statements for reporting a study to elicit parameter values and/or distributions and 11 statements for reporting a Delphi survey to obtain expert opinion. Fifty experts were invited to become members of the Delphi process panel by e-mail. Data analysis summarised the extent of agreement (using a pre-defined 75 % ‘consensus’ threshold) on the definitions and suggested reporting criteria. Free-text comments were analysed using thematic analysis.
Results
The final panel comprised 12 experts. Consensus was achieved for the definitions of expert (88 %); expert parameter values (83 %); and expert elicitation study (83 %). The panel recommended criteria to use when reporting an expert elicitation study (16 criteria) and a Delphi study to collate expert opinion (11 criteria).
Conclusion
This study has produced guidelines for reporting two types of study design to use expert judgement in model-based EE: (i) an expert elicitation study requiring 16 reporting criteria; and (ii) a Delphi study to collate expert opinion requiring 11 reporting criteria.

Similar content being viewed by others
References
Dakin H, Devlin N, Feng Y, Rice N, O’Neill P, Parkin D. The influence of cost-effectiveness and other factors on NICE decisions. Health Econ. 2015;24(10):1256–71.
Brennan A, Chick SE, Davies R. A taxonomy of model structures for economic evaluation of health technologies. Health Econ. 2006;15(12):1295–310.
Excellence NIfC. Guide to the methods of technology appraisal. London: NICE; 2004.
Sculpher MJ, Claxton K, Drummond M, McCabe C. Whither trial-based economic evaluation for health care decision making? Health Econ. 2006;15(7):677–87.
Marsden G, Wonderling D. Cost-effectiveness analysis: role and implications. Phlebology. 2013;28(suppl 1):135–40.
Annemans L, Genesté B, Jolain B. Early modelling for assessing health and economic outcomes of drug therapy. Value Health. 2000;3(6):427–34.
IJzerman MJ, Steuten LM. Early assessment of medical technologies to inform product development and market access. Appl Health Econ Health Policy. 2011;9(5):331–47.
Iglesias CP. Does assessing the value for money of therapeutic medical devices require a flexible approach? Expert Rev Pharmacoecon Outcomes Res. 2015;15(1):21–32.
Morgan MG. Use (and abuse) of expert elicitation in support of decision making for public policy. Proc Natl Acad Sci. 2014;111(20):7176–84. doi:10.1073/pnas.1319946111.
Kuhnert PM, Martin TG, Griffiths SP. A guide to eliciting and using expert knowledge in Bayesian ecological models. Ecol Lett. 2010;13(7):900–14. doi:10.1111/j.1461-0248.2010.01477.x.
Mullen PM. Delphi: myths and reality. J Health Organ Manag. 2003;17(1):37–52.
Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. 2000;32(4):1008–15.
Sullivan W, Payne K. The appropriate elicitation of expert opinion in economic models. Pharmacoeconomics. 2011;29(6):455.
Rauch W. The decision delphi. Technol Forecast Soc Change. 1979;15(3):159–69.
O’Hagan A, Buck CE, Daneshkhah A, Eiser JR, Garthwaite PH, Jenkinson DJ, et al. Uncertain judgements eliciting experts’ probabilities. Chichester: Wiley; 2006.
Gosling JP. Methods for eliciting expert opinion to inform health technology assessment. In: Vignette Commissioned by the MRC Methodology Advisory Group. Medical Research Council (MRC) and National Institure for Health Research (NIHR). 2014. https://www.google.co.uk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=0ahUKEwjr7Krwu_LMAhWGLsAKHbylCNUQFgghMAA&url=https%3A%2F%2Fwww.mrc.ac.uk%2Fdocuments%2Fpdf%2Fmethods-for-eliciting-expert-opinion-gosling-2014%2F&usg=AFQjCNGhWG6GGK0oAPr0IYtYCBttaC3Jnw&sig2=aRuqYgYzvqJ1ibAH8EDoHA&bvm=bv.122676328,d.ZGg. Accessed 21 June 2016.
Grigore B, Peters J, Hyde C, Stein K. Methods to elicit probability distributions from experts: a systematic review of reported practice in health technology assessment. Pharmacoeconomics. 2013;31(11):991–1003.
Evans C, Crawford B. Expert judgement in pharmacoeconomic studies. Pharmacoeconomics. 2000;17(6):545–53.
Moher D, Schulz KF, Simera I, Altman DG. Guidance for developers of health research reporting guidelines. PLoS Med. 2010;7(2):e1000217.
Jones J, Hunter D. Consensus methods for medical and health services research. BMJ. 1995;311(7001):376–80.
Johnson SR, Tomlinson GA, Hawker GA, Granton JT, Feldman BM. Methods to elicit beliefs for Bayesian priors: a systematic review. J Clin Epidemiol. 2010;63(4):355–69.
Kadane J, Wolfson LJ. Experiences in elicitation. J R Stat Soc Ser D Stat. 1998;47(1):3–19.
Kinnersley N, Day S. Structured approach to the elicitation of expert beliefs for a Bayesian-designed clinical trial: a case study. Pharm Stat. 2013;12(2):104–13.
Knol AB, Slottje P, van der Sluijs JP, Lebret E. The use of expert elicitation in environmental health impact assessment: a seven step procedure. Environ Health. 2010;9(1):19.
O’Hagan A. SHELF: the SHeffield ELicitation Framework. 2010. http://www.tonyohagan.co.uk/shelf/. Accessed 26 Feb 2016.
Morris DE, Oakley JE, Crowe JA. A web-based tool for eliciting probability distributions from experts. Environ Model Softw. 2014;52:1–4.
Morris E, Oakley J. MATCH uncertainty elicitation tool. 2014. http://optics.eee.nottingham.ac.uk/match/uncertainty.php/. Accessed 26 Feb 2016.
Cantrill J, Sibbald B, Buetow S. The Delphi and nominal group techniques in health services research. Int J Pharm Pract. 1996;4(2):67–74.
Melton LJ 3rd, Thamer M, Ray NF, Chan JK, Chesnut CH 3rd, Einhorn TA, et al. Fractures attributable to osteoporosis: report from the National Osteoporosis Foundation. J Bone Miner Res. 1997;12(1):16–23.
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ. 2013;346:f1049.
Evers S, Goossens M, De Vet H, Van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: consensus on Health Economic Criteria. Int J Technol Assess Health Care. 2005;21(02):240–5.
Ramos MCP, Barton P, Jowett S, Sutton AJ. A systematic review of research guidelines in decision-analytic modeling. Value Health. 2015;18(4):512–29.
Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user’s guide. Pharmacoeconomics. 2008;26(8):661–77.
Oxford Dictionaries. Oxford University Press. 2016. http://www.oxforddictionaries.com. Accessed 26 Feb 2016.
Garthwaite PH, Kadane JB, O’Hagan A. Statistical methods for eliciting probability distributions. J Am Stat Assoc. 2005;100(470):680–701.
The Free Dictionary. Farlex. 2016. http://www.thefreedictionary.com. Accessed 26 Feb 2016.
Macmillan Dictionary. Macmillan Publishers. 2016. http://www.macmillandictionary.com/. Accessed 26 Feb 2016.
Acknowledgments
CPI contributed to the design and the conduct of the study, commented on drafts of the manuscript and produced the final version of the manuscript. AT contributed to the design and the conduct of the study, analysed the data, commented on drafts of the manuscript and approved the final version of the manuscript. WR contributed to the design and the conduct of the study, commented on drafts of the manuscript and approved the final version of the manuscript. KP conceived the idea for this study, contributed to the design and the conduct of the study, produced a first draft of the manuscript, commented on subsequent drafts and approved the final version of the manuscript. We also want to thank the following members of the Health Economics Elicitation Group for their contribution to this study: Laura Bojke, Qi Cao, Ali Daneshkhah, Christopher Evans, Bogdan Grigore, Anthony O’Hagan, Vincent Levy, Claire Rothery, Fabrizio Ruggeri, Ken Stein, Matt Stevenson and Patricia Vella Bonanno.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No funding was received for this study and the authors of this paper, Cynthia P. Iglesias, Alexander Thompson, Wolf H. Rogowski and Katherine Payne, declare that there is no conflict of interest regarding its publication.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Iglesias, C.P., Thompson, A., Rogowski, W.H. et al. Reporting Guidelines for the Use of Expert Judgement in Model-Based Economic Evaluations. PharmacoEconomics 34, 1161–1172 (2016). https://doi.org/10.1007/s40273-016-0425-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-016-0425-9