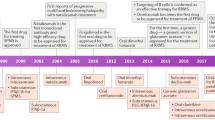
Abstract
Teriflunomide (Aubagio®), which was developed by Sanofi, is an oral immunomodulatory agent targeting the mitochondrial enzyme dihydroorotate dehydrogenase and available to adults to treat relapsing-remitting multiple sclerosis (MS). On 18 June 2021, teriflunomide received its first approval in this indication in pediatric patients aged ≥ 10 years in the EU. This article summarizes the milestones in the development of teriflunomide leading to this first pediatric approval for relapsing-remitting MS.
Similar content being viewed by others
References
Macaron G, Feng J, Moodley M, et al. Newer treatment approaches in pediatric-onset multiple sclerosis. Curr Treat Options Neurol. 2019;21(10):50.
Benson LA, Healy BC, Gorman MP, et al. Elevated relapse rates in pediatric compared to adult MS persist for at least 6 years. Mult Scler Relat Disord. 2014;3(2):186–93.
Yeh EA, Weinstock-Guttman B, Ramanathan M, et al. Magnetic resonance imaging characteristics of children and adults with paediatric-onset multiple sclerosis. Brain. 2009;132(Pt 12):3392–400.
Pfeifenbring S, Bunyan RF, Metz I, et al. Extensive acute axonal damage in pediatric multiple sclerosis lesions. Ann Neurol. 2015;77(4):655–67.
Renoux C, Vukusic S, Mikaeloff Y, et al. Natural history of multiple sclerosis with childhood onset. N Engl J Med. 2007;356(25):2603–13.
Julian L, Serafin D, Charvet L, et al. Cognitive impairment occurs in children and adolescents with multiple sclerosis: results from a United States network. J Child Neurol. 2013;28(1):102–7.
McGinley M, Rossman IT. Bringing the HEET: the argument for high-efficacy early treatment for pediatric-onset multiple sclerosis. Neurotherapeutics. 2017;14(4):985–98.
European Medicines Agency. Gilenya® (fingolimod): EU summary of product characteristics. 2021. https://www.ema.europa.eu/. Accessed 4 Aug 2021.
European Medicines Agency. Aubagio® (teriflunomide): EU summary of product characteristics. 2021. https://www.ema.europa.eu/en. Accessed 3 Sep 2021.
Genzyme. Aubagio® (teriflunomide): US prescribing information. 2021. https://www.accessdata.fda.gov/. Accessed 3 Sep 2021.
Genzyme. FDA approves Genzyme's Aubagio® (teriflunomide), a once-daily, oral treatment for relapsing multiple sclerosis [media release]. 2012. https://www.genzyme.com.
Genzyme. Genzyme announces EMA accepts oral teriflunomide marketing application for treatment of multiple sclerosis [media release]. 2012. https://www.genzyme.com.
Sanofi. European Commission approves Aubagio® (teriflunomide) as the first oral MS therapy for first-line treatment of children and adolescents living with relapsing-remitting multiple sclerosis [media release]. 2021. https://www.sanofi.com/.
Sanofi. Sanofi provides update on Aubagio® (teriflunomide) submission for children and adolescence with relapsing-remitting multiple sclerosis in the US [media release]. 2021. https://www.sanofi.com/.
Bar-Or A, Pachner A, Menguy-Vacheron F, et al. Teriflunomide and its mechanism of action in multiple sclerosis. Drugs. 2014;74(6):659–74.
Kasarello K, Cudnoch-Jedrzejewska A, Czlonkowski A, et al. Mechanism of action of three newly registered drugs for multiple sclerosis treatment. Pharmacol Rep. 2017;69(4):702–8.
European Medicines Agency. Aubagio® (teriflunomide): EU public assessment report. 2021. https://www.ema.europa.eu/. Accessed 3 Sep 2021.
Chitnis T, Banwell B, Arnold DL, et al. Teriflunomide efficacy and safety in pediatric patients with relapsing forms of MS: interim analysis of open-label TERIKIDS trial extension. Mult Scler J. 2020;26(Suppl 3):5–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Authorship and Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Julia Paik is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Paik, J. Teriflunomide: Pediatric First Approval. Pediatr Drugs 23, 609–613 (2021). https://doi.org/10.1007/s40272-021-00471-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-021-00471-1