Abstract
Pulmonary hypertension is a life-threatening condition that affects people of all ages that can occur as an idiopathic disorder at birth or as part of a variety of cardiovascular and infectious disorders. It is commonly treated with inhaled pulmonary vasodilators such as nitric oxide and less frequently using formulations and analogs of prostacyclin. To minimize systemic effects and preserve pulmonary vasodilation, vasodilators are often administered directly into the airway. Nitric oxide is the only USA Food and Drug Administration-approved inhaled pulmonary vasodilator that can be used during mechanical ventilation. Over the past two decades, interest has grown in the use of aerosolized prostacyclin and prostacyclin analogs for the treatment of pulmonary hypertension during mechanical ventilation. Clinicians who administer inhaled prostacyclin may not have a clear understanding of its risks because of the lack of data from large clinical trials examining safety and efficacy; moreover, its safe use remains poorly documented. The off-label use of drugs is legitimate, but prescribers must recognize the potential complications and liability in doing so. This manuscript aims to address potential problems related to the aerosol administration of pulmonary vasodilators in the mechanically ventilated neonatal patient.

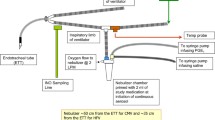
(Drawn by David White, with permission)

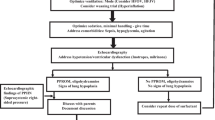
(Drawn by David White, with permission)
Similar content being viewed by others
References
Hill NS, Farber HW. Pulmonary hypertension. Totowa: Springer; 2008.
Walsh-Sukys MC, Tyson JE, Wright LL, Bauer CR, Korones SB, Stevenson DK, et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatrics. 2000;105:14–20.
INOmax®. Nitric oxide gas. 2015. http://inomax.com/wp-content/uploads/2016/02/INOmax-PI-web-2015-10.pdf. Accessed 30 Mar 2016.
American Academy of Pediatrics Committee on Drugs. Unapproved uses of approved drugs: the physician, the package insert, and the Food and Drug Administration: subject review. Pediatrics 1996;98:143–5.
Off-label use of drugs in children. Pediatrics. 2014;133(3):563–7.
Ozek E, Soll R, Schimmel MS. Partial exchange transfusion to prevent neurodevelopmental disability in infants with polycythemia. Cochrane Database Syst Rev. 2010:CD005089.
Rabinovitch M. Morphology of the developing pulmonary bed: pharmacologic implications. Pediatr Pharmacol (New York). 1985;5:31–48.
Levin DL. Morphologic analysis of the pulmonary vascular bed in congenital left-sided diaphragmatic hernia. J Pediatr. 1978;92:805–9.
Kitagawa M, Hislop A, Boyden EA, Reid L. Lung hypoplasia in congenital diaphragmatic hernia. A quantitative study of airway, artery, and alveolar development. Br J Surg. 1971;58:342–6.
Naeye RL, Shochat SJ, Whitman V, Maisels MJ. Unsuspected pulmonary vascular abnormalities associated with diaphragmatic hernia. Pediatrics. 1976;58:902–6.
Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:13S–24S.
Philips JB 3rd, Lyrene RK, Godoy G, Graybar G, Barefield E, Sams JE, et al. Hemodynamic responses of chronically instrumented piglets to bolus injections of group B streptococci. Pediatr Res. 1988;23:81–5.
Shankaran S, Farooki ZQ, Desai R. beta-hemolytic streptococcal infection appearing as persistent fetal circulation. Am J Dis Child. 1982;136:725–7.
Gersony WM, Duc GV, Sinclair JC. “PFC” syndrome (persistence of the fetal circulation). Circulation 1969;40:Suppl III-87.
Grover RF, Reeves JT, Blount SG Jr. Tolazoline hydrochloride (Priscoline): an effective pulmonary vasodilator. Am Heart J. 1961;61:5–15.
Ward RM. Pharmacology of tolazoline. Clin Perinatol. 1984;11:703–13.
Goetzman BW, Sunshine P, Johnson JD, Wennberg RP, Hackel A, Merten DF, et al. Neonatal hypoxia and pulmonary vasospasm: response to tolazoline. J Pediatr. 1976;89:617–21.
Stevens DC, Schreiner RL, Bull MJ, Bryson CQ, Lemons JA, Gresham EL, et al. An analysis of tolazoline therapy in the critically-ill neonate. J Pediatric Surg. 1980;15:964–70.
Stevenson DK, Kasting DS, Darnall RA Jr, Ariagno RL, Johnson JD, Malachowski N, et al. Refractory hypoxemia associated with neonatal pulmonary disease: the use and limitations of tolazoline. J Pediatr. 1979;95:595–9.
Ward RM, Daniel CH, Kendig JW. Oliguria and tolazoline pharmacokinetics in the newborn. Pediatrics. 1986;77:307–15.
Andrews AF, Roloff DW, Bartlett RH. Use of extracorporeal membrane oxygenators in persistent pulmonary hypertension of the newborn. Clin Perinatol. 1984;11:729–35.
Beck R, Anderson KD, Pearson GD, Cronin J, Miller MK, Short BL. Criteria for extracorporeal membrane oxygenation in a population of infants with persistent pulmonary hypertension of the newborn. J Pediatric Surg. 1986;21:297–302.
Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA. 1987;84:9265–9.
Ignarro LJ, Byrns RE, Buga GM, Wood KS. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987;61:866–79.
Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–6.
Kinsella JP, Abman SH. Recent developments in the pathophysiology and treatment of persistent pulmonary hypertension of the newborn. J Pediatr. 1995;126:853–64.
Gryglewski RJ. Prostacyclin among prostanoids. Pharmacol Rep. 2008;60:3–11.
Moncada S, Gryglewski R, Bunting S, Vane JR. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976;263:663–5.
Van Heerden PV, Webb SA, Hee G, Corkeron M, Thompson WR. Inhaled aerosolized prostacyclin as a selective pulmonary vasodilator for the treatment of severe hypoxaemia. Anaesth Intensive Care. 1996;24:87–90.
Davis MD, Hunt J. Exhaled breath condensate pH assays. Immunol Allergy Clin N Am. 2012;32:377–86.
United States Food and Drug Administration Center for Drug Evaluation and Research Approval Package for Iloprost. Submission Number 21779. 2004.
United States Food and Drug Administration Center for Drug Evaluation and Research Approval Package for Treprostinil. Submission Number 22387. 2009.
Actelion. Ventavis prescribing information. South San Francisco: Actelion; 2013
United Therapeutics Corp. Tyvaso prescribing information. Research Triangle Park: United Therapeutics Corp; 2016.
Horen B, Montastruc JL, Lapeye-Mestre M. Adverse drug reactions and off-label drug use in paediatric outpatients. Br J Clin Pharmacol. 2002;54(6):665–70.
GSK. Flolan prescribing information. Research Triangle Park: GSK; 2008.
Yilmiz O, Kahyeci Zeybek C, et al. Inhaled iloprost in preterm infants with severe respiratory distress syndrome and pulmonary hypertension. Am J Perinatol. 2014;31:321–6.
Tissot C, Beghetti M. Review of inhaled iloprost for the control of pulmonary artery hypertension in children. Vasc Health Risk Manag. 2009;5:325–31.
Brown A, Gillespie J, Miquel-Vergas F, et al. Inhaled epoprostenol therapy for pulmonary hypertension: Improves oxygenation index more consistently in neonates than in older children. Pulmon Circ. 2012;2:61–6.
Cosa N, Costa E. Inhaled pulmonary vasodilators for persistent pulmonary hypertension of the newborn: safety issues relating to drug administration and delivery devices. Med Dev Evid Res. 2016;9:45–51.
Institute for Safe Medication Practices (IMSP). ISMP list of high-alert medications in acute care settings. 2014. https://www.ismp.org/tools/institutionalhighAlert.asp. Accessed 30 Mar 2016.
Kingman MS, Tankersley MA, Lombardi S, Spence S, Torres F, Chin KS, et al. Prostacyclin administration errors in pulmonary arterial hypertension patients admitted to hospitals in the United States: a national survey. J Heart Lung Transplant. 2010;29:841–6.
Dunkley KA, Louzon PR, Lee J, Vu S. Efficacy, safety, and medication errors associated with the use of inhaled epoprostenol for adults with acute respiratory distress syndrome: a pilot study. Ann Pharmacother. 2013;47:790–6.
Siobal MS, Kallet RH, Pittet JF, Warnecke EL, Kraemer RW, Venkayya RV, et al. Description and evaluation of a delivery system for aerosolized prostacyclin. Respir Care. 2003;48:742–53.
Actelion. Veletri prescribing information. South San Francisco: Actelion; 1995.
Luk CK, Dulfano MJ. Effect of pH, viscosity and ionic-strength changes on ciliary beating frequency of human bronchial explants. Clin Sci (Lond). 1983;64:449–51.
Ricciardolo FL, Gaston B, Hunt J. Acid stress in the pathology of asthma. J Allergy Clin Immunol. 2004;113:610–9.
Holma B, Lindegren M, Andersen JM. pH effects on ciliomotility and morphology of respiratory mucosa. Arch Environ Health. 1977;32:216–26.
Le Brun PP, de Boer AH, Heijerman HG, Frijlink HW. A review of the technical aspects of drug nebulization. Pharm World Sci. 2000;22:75–81.
Van Heerden PV, Blythe D, Webb SA. Inhaled aerosolized prostacyclin and nitric oxide as selective pulmonary vasodilators in ARDS—a pilot study. Anaesth Intensive Care. 1996;24:564–8.
Berlinski A, Willis JR. Albuterol delivery by 4 different nebulizers placed in 4 different positions in a pediatric ventilator in vitro model. Respir Care. 2013;58:1124–33.
Cancado JE, Mendes ES, Arana J, Horvath G, Monzon ME, Salathe M, et al. Effect of airway acidosis and alkalosis on airway vascular smooth muscle responsiveness to albuterol. BMC Pharmacol Toxicol. 2015;16:9.
Clary-Meinesz C, Mouroux J, Cosson J, Huitorel P, Blaive B. Influence of external pH on ciliary beat frequency in human bronchi and bronchioles. Eur Respir J. 1998;11:330–3.
Horvath G, Schmid N, Fragoso MA, Schmid A, Conner GE, Salathe M, et al. Epithelial organic cation transporters ensure pH-dependent drug absorption in the airway. Am J Respir Cell Mol Biol. 2007;36:53–60.
Shin HW, Shelley DA, Henderson EM, Fitzpatrick A, Gaston B, George SC. Airway nitric oxide release is reduced after PBS inhalation in asthma. J Appl Physiol. 2007;102:1028–33.
Hunt JF, Fang K, Malik R, Snyder A, Malhotra N, Platts-Mills TA, et al. Endogenous airway acidification. Implications for asthma pathophysiology. Am J Respir Crit Care Med. 2000;161:694–9.
Eichelbronner O, Reinelt H, Wiedeck H, Mezody M, Rossaint R, Georgieff M, et al. Aerosolized prostacyclin and inhaled nitric oxide in septic shock—different effects on splanchnic oxygenation? Intensive Care Med. 1996;22:880–7.
Davis MD, Walsh BK, Dwyer ST, et al. Safety of an alkalinizing buffer designed for inhaled medications in humans. Respir Care. 2013;58(7):1226–32.
Siobal M. Aerosolized prostacyclins. Respir Care. 2004;49:640–52.
Sun FF, Taylor BM. Metabolism of prostacyclin in rat. Biochemistry. 1978;17:4096–101.
Rosenkranz B, Fischer C, Frolich JC. Prostacyclin metabolites in human plasma. Clin Pharmacol Ther. 1981;29:420–4.
Nephron Pharmaceuticals. Albuterol sulfate prescribing information. Orlando: Nephron Pharmaceuticals.
Nephron Pharmaceuticals. Ipratropium bromide prescribing information. Orlando: Nephron Pharmaceuticals.
AstraZeneca. Pulmicort Respule prescribing information. Sodertalji: AstraZeneca; 2010.
Hospira, Inc. Acetylcysteine prescribing information. Lake Forest: Hospira, Inc; 2004.
Genentech, Inc. Pulmozyme prescribing information. South San Francisco: Genentech, Inc; 2014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Robert Ward, MD, Steven Donn, MD, and Michael D. Davis have served as paid consultants to Mallinckrodt Pharmaceuticals (formally Ikaria, Inc.). Mallinckrodt Pharmaceuticals had no input into the content of this review and had no editorial involvement with the content of this manuscript. None of the authors own stock or serve as employees of companies with an interest in this paper.
Rights and permissions
About this article
Cite this article
Davis, M.D., Donn, S.M. & Ward, R.M. Administration of Inhaled Pulmonary Vasodilators to the Mechanically Ventilated Neonatal Patient. Pediatr Drugs 19, 183–192 (2017). https://doi.org/10.1007/s40272-017-0221-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40272-017-0221-9