Abstract
Background
Intranasal insulin is a potential treatment for neurodegenerative disease shown to increase cerebral glucose uptake, reduce amyloid plaques, and improve verbal memory in cognitively impaired as well as healthy adults. Investigations have suggested rapid-acting insulins such as glulisine may result in superior cognitive benefits compared with regular insulin.
Objective
The aim of this study was to evaluate the safety and efficacy of rapid-acting intranasal glulisine in subjects with amnestic mild cognitive impairment (MCI) or mild probable Alzheimer’s disease (AD).
Methods
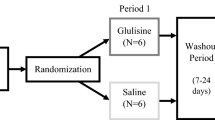
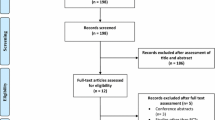
We performed a single-center, randomized, double-blind, placebo-controlled study to evaluate the efficacy of intranasal glulisine 20 IU twice daily versus saline placebo in 35 memory-impaired (MCI/AD) subjects using the Impel NeuroPharma I109 Precision Olfactory Delivery (POD®) device. The 13-item Alzheimer’s Disease Assessment Scale–Cognitive Subscale (ADAS-Cog13), Clinical Dementia Rating (CDR) global score, and Functional Assessment Questionnaire (FAQ) were measured at baseline and 3 and 6 months. Secondary outcome measures included digit span forward/backwards, Trail Making Test Parts A/B, Controlled Oral Word Association Test (COWAT), and Weschler Memory Scale (WMS)-IV logical memory. Adverse effects (AEs) and serious adverse effects (SAEs) were measured along with blood glucose/insulin levels.
Results
No significant difference in ADAS-Cog13, CDR Sum of Boxes (CDR-SOB), or FAQ scores were found between treatment groups at 3 and 6 months. Subjects in the saline group were significantly older than those in the glulisine group (p = 0.022). No significant differences in sex, education, apolipoprotein E4 (ApoE4) status, and Montreal Cognitive Assessment (MoCA) score existed between treatment groups. Overall, the number of adverse events per person was similar between groups (2.32 vs. 2.24; p = 0.824), although subjects receiving intranasal glulisine had higher rates of nasal irritation (25.0% vs. 13.9%) and respiratory symptoms (15.9% vs. 8.3%) compared with placebo. There were no differences in blood sugar or rate of hypoglycemia between the treatment and placebo groups.
Conclusions
Intranasal glulisine was relatively safe and well-tolerated and did not consistently impact peripheral glucose or insulin levels. There were no enhancing effects of intranasal glulisine on cognition, function, or mood, but the ability to detect significance was limited by the number of subjects successfully enrolled and the study duration.
ClinicalTrials.gov Registration
NCT02503501.

Similar content being viewed by others
References
Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Dement. 2020. https://doi.org/10.1002/alz.12068 ((Epub 10 March 2020)).
Kellar D, Craft S. Brain insulin resistance in Alzheimer’s disease and related disorders: mechanisms and therapeutic approaches. Lancet Neurol. 2020;19(9):758–66.
Laws SM, Gaskin S, Woodfield A, et al. Insulin resistance is associated with reductions in specific cognitive domains and increases in CSF tau in cognitively normal adults. Sci Rep. 2017;7(1):9766.
Yarchoan M, Toledo JB, Lee EB, et al. Abnormal serine phosphorylation of insulin receptor substrate 1 is associated with tau pathology in Alzheimer’s disease and tauopathies. Acta Neuropathol. 2014;128(5):679–89.
Ott A, Stolk RP, van Harskamp F, et al. Diabetes mellitus and the risk of dementia: the rotterdam study. Neurology. 1999;53(9):1937–42.
Steen E, Terry BM, Rivera EJ, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease—is this type 3 diabetes? J Alzheimers Dis. 2005;7(1):63–80.
Dhuria SV, Hanson LR, Frey WH 2nd. Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci. 2010;99(4):1654–73.
Craft S, Asthana S, Cook DG, et al. Insulin dose-response effects on memory and plasma amyloid precursor protein in Alzheimer’s disease: interactions with apolipoprotein E genotype. Psychoneuroendocrinology. 2003;28(6):809–22.
Craft S, Baker LD, Montine TJ, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69(1):29–38.
Benedict C, Hallschmid M, Schmitz K, et al. Intranasal insulin improves memory in humans: superiority of insulin aspart. Neuropsychopharmacology. 2007;32(1):239–43.
Rosenbloom MH, Barclay TR, Pyle M, et al. A single-dose pilot trial of intranasal rapid-acting insulin in apolipoprotein E4 carriers with mild-moderate Alzheimer’s disease. CNS Drugs. 2014;28(12):1185–9.
Reger MA, Watson GS, Frey WH 2nd, et al. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging. 2006;27(3):451–8.
Reger MA, Watson GS, Green PS, et al. Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J Alzheimers Dis. 2008;13(3):323–31.
Ito K, Hutmacher MM. Predicting the time to clinically worsening in mild cognitive impairment patients and its utility in clinical trial design by modeling a longitudinal clinical dementia rating sum of boxes from the ADNI database. J Alzheimers Dis. 2014;40(4):967–79.
Evans S, McRae-McKee K, Wong MM, et al. The importance of endpoint selection: how effective does a drug need to be for success in a clinical trial of a possible Alzheimer’s disease treatment? Eur J Epidemiol. 2018;33(7):635–44.
Claxton A, Baker LD, Hanson A, et al. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia. J Alzheimers Dis. 2015;44(3):897–906.
Valla J, Yaari R, Wolf AB, et al. Reduced posterior cingulate mitochondrial activity in expired young adult carriers of the APOE epsilon4 allele, the major late-onset Alzheimer’s susceptibility gene. J Alzheimers Dis. 2010;22(1):307–13.
Craft S, Raman R, Chow TW, et al. Safety, efficacy, and feasibility of intranasal insulin for the treatment of mild cognitive impairment and alzheimer disease dementia: a randomized clinical trial. JAMA Neurol. 2020;77(9):1099–109.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The study was supported by donations raised by the Regions Hospital Foundation.
Conflicts of interest
All authors declare having no conflicts of interest in connection with this work.
Ethics Approval
The study was approved by the Health Partners Institute IRB.
Consent to participate
Consent was obtained from all participants.
Availability of data and material
Not applicable
Code availability
Not applicable.
Author Contributions
Michael Rosenbloom, William Frey, Leah Hanson: study design, composition of manuscript and revision of manuscript; Terry Barclay: study design, neuropsychiatric testing and revision of manuscript; Bhavani Kashyap: project coordination and neuropsychometric testing; Lyndsay Hage: project coordination and subject recruitment; Lauren O’Keefe: data analysis and revision of manuscript; Aleta Svitak: data management; Maria Pyle: project coordination and subject recruitment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rosenbloom, M., Barclay, T.R., Kashyap, B. et al. A Phase II, Single-Center, Randomized, Double-Blind, Placebo-Controlled Study of the Safety and Therapeutic Efficacy of Intranasal Glulisine in Amnestic Mild Cognitive Impairment and Probable Mild Alzheimer’s Disease. Drugs Aging 38, 407–415 (2021). https://doi.org/10.1007/s40266-021-00845-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-021-00845-7