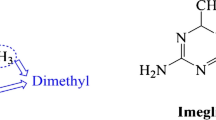Abstract
Imeglimin hydrochloride (TWYMEEG®; hereafter referred to as imeglimin) is an orally administered, first-in-class glimin being developed by Poxel and, in several Asian countries, Sumitomo Dainippon Pharma for the treatment of type 2 diabetes (T2D). The glimins are a novel class of glucose-lowering agents that target multiple components of diabetes-associated pathology. In June 2021, imeglimin received its first approval for use in T2D in Japan. The Japanese approval was based on extensive preclinical and clinical data, including positive results from the pivotal phase III TIMES programme. This article summarizes the milestones in the development of imeglimin leading to this first approval for T2D.
Similar content being viewed by others
References
Hallakou-Bozec S, Vial G, Kergoat M, et al. Mechanism of action of imeglimin: a novel therapeutic agent for type 2 diabetes. Diabetes Obes Metab. 2021;23(3):664–73.
Konkwo C, Perry RJ. Imeglimin: current development and future potential in type 2 diabetes. Drugs. 2021;81(2):185–90.
Vuylsteke V, Chastain LM, Maggu GA, et al. Imeglimin: a potential new multi-target drug for type 2 diabetes. Drugs R D. 2015;15(3):227–32.
Poxel. Imeglimin hydrochloride (TWYMEEG®): Japanese prescribing information 2021. http://www.pmda.go.jp/. Accessed 23 Jul 2021.
Poxel. Poxel and Sumitomo Dainippon Pharma announce the approval of TWYMEEG(Rm) (imeglimin hydrochloride) for the treatment of type 2 diabetes in Japan [media release]. 23 Jun 2021.
Poxel. Poxel announces submission of imeglimin Japanese New Drug Application for the treatment of type 2 diabetes by Sumitomo Dainippon Pharma [media release]. 30 Jul 2020.
Sumitomo Dainippon Pharma, Poxel SA. Sumitomo Dainippon Pharma and Poxel announce strategic partnership for development and commercialization of imeglimin, an investigational therapeutic agent for type 2 diabetes, in Japan, China and eleven other Asian countries [media release]. 30 Oct 2017.
Roivant Sciences, Poxel SA. Roivant and Poxel announce strategic agreement for development and commercialization of imeglimin in the U.S., Europe, and additional countries worldwide [media release]. 12 Feb 2018.
Poxel. Poxel regains imeglimin rights from Metavant [media release]. 14 Jan 2021.
Vial G, Lamarche F, Cottet-Rousselle C, et al. The mechanism by which imeglimin inhibits gluconeogenesis in rat liver cells. Endocrinol Diab Metab. 2021;4(2):e00211.
Hallakou-Bozec S, Kergoat M, Fouqueray P, et al. Imeglimin amplifies glucose-stimulated insulin release from diabetic islets via a distinct mechanism of action. PLoS ONE. 2021;16(2):e0241651.
Lablanche S, Tubbs E, Cottet-Rousselle C, et al. Imeglimin protects INS-1 cells and human islets against high glucose-and high fructose-induced cell death by inhibiting the mitochondrial PTP opening [abstract no. 81-OR]. Diabetes. 2018;67 (Suppl 1):A21.
Hallakou-Bozec S, Kergoat M, Moller DE, et al. Imeglimin preserves islet β-cell mass in type 2 diabetic ZDF rats. Endocrinol Diabetes Metab. 2021;4(2):e00193.
Perry RJ, Cardone RL, Petersen MC, et al. Imeglimin lowers glucose primarily by amplifying glucose-stimulated insulin secretion in high-fat-fed rodents. Am J Physiol Endocrinol Metab. 2016;311(2):E461–70.
Vial G, Chauvin MA, Bendridi N, et al. Imeglimin normalizes glucose tolerance and insulin sensitivity and improves mitochondrial function in liver of a high-fat, high-sucrose diet mice model. Diabetes. 2015;64(6):2254–64.
Lachaux M, Soulie M, Hamzaoui M, et al. Short-and long-term administration of imeglimin counters cardiorenal dysfunction in a rat model of metabolic syndrome. Endocrinol Diabetes Metab. 2020;3(3):e00128.
Pacini G, Mari A, Fouqueray P. Imeglimin increases glucose-dependent insulin secretion and improves beta-cell function in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17(6):541–5.
Dubourg J, Perrimond-Dauchy S, Felices M, et al. Absence of QTc prolongation in a thorough QT study with imeglimin, a first in class oral agent for type 2 diabetes mellitus. Eur J Clin Pharmacol. 2020;76(10):1393–400.
Clemence C, Fouqueray P, Sebastien B. In vitro investigation, pharmacokinetics, and disposition of imeglimin, a novel oral antidiabetic drug, in preclinical species and humans. Drug Metab Dispos. 2020;48(12):1330–46.
Chevalier C, Perrimond-Dauchy S, Dubourg J, et al. Lack of drug-drug interaction between cimetidine, a renal transporter inhibitor, and imeglimin, a novel oral antidiabetic drug, in healthy volunteers. Eur J Drug Metab Pharmacokinet. 2020;45(6):725–33.
Fouqueray P, Perrimond-Dauchy S, Bolze S. Imeglimin does not induce clinically relevant pharmacokinetic interactions when combined with either metformin or sitagliptin in healthy subjects. Clin Pharmacokinet. 2020;59(10):1261–71.
Chevalier C, Dubourg J, Bolze S, et al. Pharmacokinetics of imeglimin in subjects with moderate hepatic impairment. Clin Pharmacokinet. 2021;60(4):485–90.
Dubourg J, Fouqueray P, Thang C. Efficacy and safety of imeglimin monotherapy versus placebo in Japanese patients with type 2 diabetes (TIMES 1): a double-blind, randomized, placebo-controlled, parallel-group, multicenter phase 3 trial. Diabetes Care. 2021;44:952–9.
Kaku K, Dubourg J, Thang C, et al. Long-term treatment with imeglimin as add-on to oral antidiabetes therapy in Japanese patients with type 2 diabetes (Times 2). Diabetologia. 2020;63(Suppl 1):S306.
Dubourg J, Watada H, Thang C, et al. Efficacy and safety of imeglimin in combination with insulin in Japanese patients with type 2 diabetes: results of TIMES 3 trial [abstract no. 637]. Diabetologia. 2020;63(Suppl 1):S307.
Dubourg J, Ueki K, Grouin JM, et al. Efficacy and safety of imeglimin in Japanese patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled, dose-ranging phase 2b trial. Diabetes Obes Metab. 2021;23(3):800–10.
Fouqueray P, Bolze S, Pirags V, et al. Dose-ranging study to determine the optimum dose for imeglimin, a novel treatment for type 2 diabetes [abstract no. 1169-P plus poster]. Diabetes. 2015;64:A301.
Fouqueray P, Bolze S, Pirags V, et al. Imeglimin, a new oral anti-hyperglycaemic agent controls fasting and post-prandial glucose through an improvement in both insulin secretion and insulin sensitivity [poster] In: World Congress on Insulin Resistance, Diabetes and Cardiovascular Disease. 2015.
Fouqueray P, Pirags V, Diamant M, et al. The efficacy and safety of imeglimin as add-on therapy in patients with type 2 diabetes inadequately controlled with sitagliptin monotherapy. Diabetes Care. 2014;10.
Fouqueray P, Pirags V, Inzucchi SE, et al. The efficacy and safety of imeglimin as add-on therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy. Diabetes Care. 2013;36(3):565–8.
Pirags V, Lebovitz H, Fouqueray P. Imeglimin, a novel glimin oral antidiabetic, exhibits a good efficacy and safety profile in type 2 diabetic patients. Diabetes Obes Metab. 2012;14(9):852–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Yvette Lamb is a salaried employee of Adis International Ltd/Springer Nature, and declares no relevant conflicts of interest. All authors contributed to the review and are responsible for the article content.
Ethics approval, Consent to participate, Consent to publish, Availability of data and material, Code availability
Not applicable.
Additional information
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lamb, Y.N. Imeglimin Hydrochloride: First Approval. Drugs 81, 1683–1690 (2021). https://doi.org/10.1007/s40265-021-01589-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-021-01589-9