Abstract
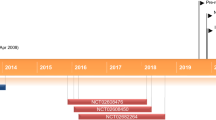
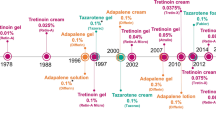
Topical trifarotene (Aklief®) is a first-in-class, fourth-generation retinoid [selective retinoic acid receptor (RAR)-γ agonist] being developed by Galderma Research and Development LLC for the treatment of acne vulgaris. In October 2019 trifarotene received its first global approval in the USA for the topical treatment of acne vulgaris in patients 9 years of age and older. This article summarizes the milestones in the development of trifarotene leading to its first global approval for acne vulgaris.
Similar content being viewed by others
References
Chien AL. Retinoids in acne management: review of current understanding, future considerations, and focus on topical treatments. J Drugs Dermatol. 2018;17(12 Suppl):s51–5.
Kolli SS, Pecone D, Pona A, et al. Topical retinoids in acne vulgaris: a systematic review. Am J Clin Dermatol. 2019;20(3):345–65.
US National Institue of Health. Lamellar ichthyosis. 2019. http://ghr.nlm.nih.gov/condition/lamellar-ichthyosis#diagnosis. Accessed 15 Oct 2019.
Vahlquist A, Fischer J, Törmä H. Inherited nonsyndromic ichthyoses: an update on pathophysiology, diagnosis and treatment. Am J Clin Dermatol. 2018;19(1):51–66.
FDA. Novel drug approvals for 2019. 2019. http://www.fda.gov. Accessed 15 Oct 2019.
Galderma. Aklief® (Trifarotene): US Prescribing Information. 2019. http://www.galderma.com/. Accessed 2019 Oct 8.
Galderma. Galderma trifarotene development program meets key milestone for lamellar ichthyosis orphan disease [media release]. 2016. http://www.galderma.com/. Accessed 15 Oct 2019.
Aubert J, Piwnica D, Bertino B, et al. Nonclinical and human pharmacology of the potent and selective topical retinoic acid receptor-gamma agonist trifarotene. Br J Dermatol. 2018;179(2):442–56.
Thoreau E, Arlabosse J, Bouix-Peter C, et al. Structure-based design of trifarotene, a potent and selective RAR agonist for the treatment of acne. Bioorg Med Chem Lett. 2018;128(101736-41).
Tan J, Thiboutot D, Popp G, et al. Randomized phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019;80(6):1691–9.
Blume-Peytavi U, Fowler J, Kemény L, et al. Long-term safety and efficacy of trifarotene 50 μg/g cream, a first-in-class RAR-γ selective topical retinoid, in patients with moderate facial and truncal acne. J Eur Acad Dermatol Venereol. 2019. https://doi.org/10.1111/jdv.15794.
Galderma (2019) Data on file
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflicts of interest
During the peer review process the manufacturer of the agent under review was offered an opportunity to comment on the article. Changes resulting from any comments received were made by the authors on the basis of scientific completeness and accuracy. Lesley Scott is a salaried employee of Adis International Ltd/Springer Nature, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
Enhanced material
For this AdisInsight Report can be found at https://doi.org/10.6084/m9.figshre.9978725.
This profile has been extracted and modified from the AdisInsight database. AdisInsight tracks drug development worldwide through the entire development process, from discovery, through pre-clinical and clinical studies to market launch and beyond.
Rights and permissions
About this article
Cite this article
Scott, L.J. Trifarotene: First Approval. Drugs 79, 1905–1909 (2019). https://doi.org/10.1007/s40265-019-01218-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-019-01218-6