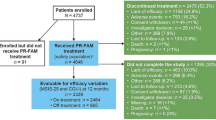
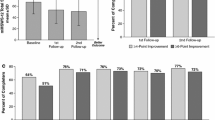
Abstract
Oral fampridine prolonged release (PR) [Fampyra®] is a lipid-soluble selective potassium channel blocker that is approved in the EU for the improvement of walking in adult multiple sclerosis (MS) patients with walking disability (expanded disability status scale score of 4–7). In clinical trials (MS-F203 and MS-F204) using an objective measure of walking improvement [the timed 25-foot walk (T25FW)], more than one-third of patients receiving fampridine PR achieved a consistent on-treatment improvement in walking speed (i.e. became TW responders) over 9–14 weeks of treatment. Fampridine PR recipients who fulfilled the definition of TW responder had mean improvements of ≈25% from baseline in T25FW walking speed. In a clinical trial (ENHANCE) that used a patient-rated measure of walking improvement [12-item MS walking scale (MSWS-12)], a significantly greater proportion of fampridine PR recipients than placebo recipients achieved a ≥8-point improvement on the MSWS-12 with 24 weeks of treatment. Where reported, adverse events were mostly mild or moderate in severity, and generally consistent with the underlying disease or mechanism of action of fampridine PR. Fampridine PR is a useful treatment option to consider in adult MS patients with walking disability.
Similar content being viewed by others
References
Gross R, Lublin F. Multiple sclerosis: an overview. In: Miller A, editor. Handbook of relapsing-remitting multiple sclerosis. Cham: Springer International Publishing; 2017. p. 1–16.
National Multiple Sclerosis Society. Multiple sclerosis: just the facts. 2016. https://www.nationalmssociety.org. Accessed 10 July 2017.
World Health Organization. Neurological disorders: public health challenges. 2006. http://www.who.int. Accessed 10 July 2017.
Horng S, Fabian M. The pathophysiology and clinical presentation of multiple sclerosis. In: Miller A, editor. Handbook of relapsing-remitting multiple sclerosis. Cham: Springer International Publishing; 2017. p. 17–40.
European Medicines Agency. Fampyra 10 mg prolonged-release tablets: EU summary of product characteristics. 2017. http://www.ema.europa.eu. Accessed 22 Aug 2017.
European Medicines Agency. European public assessment reports. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/landing/epar_search.jsp&mid=WC0b01ac058001d125. Accessed 10 July 2017.
European Medicines Agency. Fampyra: EPAR assessment report. 2011. http://www.ema.europa.eu. Accessed 10 July 2017.
The Multiple Sclerosis Society of Ireland. Fampyra (prolonged release fampridine tablets) information sheet. 2015. http://ms-society.ie. Accessed 10 July 2017.
van Diemen HA, Polman CH, van Dongen MM, et al. 4-Aminopyridine induces functional improvement in multiple sclerosis patients: a neurophysiological study. J Neurol Sci. 1993;116(2):220–6.
Fujihara K, Miyoshi T. The effects of 4-aminopyridine on motor evoked potentials in multiple sclerosis. J Neurol Sci. 1998;159(1):102–6.
van Diemen HA, Polman CH, Koetsier JC, et al. 4-Aminopyridine in patients with multiple sclerosis: dosage and serum level related to efficacy and safety. Clin Neuropharmacol. 1993;16(3):195–204.
Schwid SR, Petrie MD, McDermott MP, et al. Quantitative assessment of sustained-release 4-aminopyridine for symptomatic treatment of multiple sclerosis. Neurology. 1997;48(4):817–21.
Goodman AD, Cohen JA, Cross A, et al. Fampridine-SR in multiple sclerosis: a randomized, double-blind, placebo-controlled, dose-ranging study. Mult Scler. 2007;13(3):357–68.
March B, Cardi T. Assessment of the cardiac safety of fampridine-SR sustained-release tablets in a thorough QT/QTc evaluation at therapeutic and supratherapeutic doses in healthy individuals. Expert Opin Investig Drugs. 2009;18(12):1807–15.
Weir S, Gao Y, Henney HR 3rd. Population pharmacokinetics and pharmacodynamics of dalfampridine-ER in healthy volunteers and in patients with multiple sclerosis. Curr Med Res Opin. 2013;29(12):1637–45.
Hu X, Mehta LR, Prasad P. Population pharmacokinetic analysis of fampridine in Japanese patients with multiple sclerosis in a phase 3 study [abstract no. I-45 plus poster]. In: 25th Population Approach in Europe Meeting. 2016.
Goodman AD, Brown TR, Krupp LB, et al. Sustained-release oral fampridine in multiple sclerosis: a randomised, double-blind, controlled trial. Lancet. 2009;373(9665):732–8.
Goodman AD, Brown TR, Edwards KR, et al. A phase 3 trial of extended release oral dalfampridine in multiple sclerosis. Ann Neurol. 2010;68(4):494–502.
Hobart J, Ziemssen T, Feys P, et al. Sustained clinically meaningful improvements in walking ability with prolonged-release fampridine: results from the placebo-controlled ENHANCE study [abstract no. 254]. In: 32nd Congress of ECTRIMS. 2016.
Ziemssen T, Hobart J, Feys P, et al. Long-term efficacy and safety of prolonged-release fampridine treatment in patients with multiple sclerosis: design of the multicentre, randomised, double-blind, placebo-controlled ENHANCE study [abstract no. F2082 plus poster]. Eur J Neurol. 2015;22(Suppl 1):621.
Hobart J, Ziemssen T, Feys P, et al. Prolonged-release fampridine induces sustained clinically meaningful improvements in walking ability in people with multiple sclerosis: results from the ENHANCE trial [abstract no. P6.364]. Neurol. 2017;88(16 Suppl).
European Medicines Agency. Fampyra-H-C-2097-II-0036-G: EPAR assessment report: variation. 2017. http://www.ema.europa.eu. Accessed 10 July 2017.
Biogen. Biogen’s FAMPYRA® granted standard marketing authorization in European Union for improvement of walking in people with MS [media release]. 24 May 2017. https://www.biogen.com.
Goodman AD, Bethoux F, Brown TR, et al. Long-term safety and efficacy of dalfampridine for walking impairment in patients with multiple sclerosis: results of open-label extensions of two phase 3 clinical trials. Mult Scler. 2015;21(10):1322–31.
Goodman AD, Brown TR, Cohen JA, et al. Dose comparison trial of sustained-release fampridine in multiple sclerosis. Neurology. 2008;71(15):1134–41.
Hupperts R, Lycke J, Short C, et al. Prolonged-release fampridine and walking and balance in MS: randomised controlled MOBILE trial. Mult Scler. 2016;22(2):212–21.
Saida T, Yokoyama K, Masaki K, et al. Fampridine and walking speed in Japanese patients with MS: MOTION-JAPAN study part A results [abstract no. P-10]. In: 28th Annual meeting of the Japanese Society for Neuroimmunology. 2016.
Zörner B, Filli L, Reuter K, et al. Prolonged-release fampridine in multiple sclerosis: improved ambulation effected by changes in walking pattern. Mult Scler. 2016;22(11):1463–75.
Filli L, Zörner B, Kapitza S, et al. Monitoring long-term efficacy of fampridine in gait-impaired patients with multiple sclerosis. Neurology. 2017;88(9):832–41.
Coleman CI, Sobieraj DM, Marinucci LN. Minimally important clinical difference of the timed 25-foot walk test: results from a randomized controlled trial in patients with multiple sclerosis. Curr Med Res Opin. 2012;28(1):49–56.
Goodman AD, Brown TR, Schapiro RT, et al. A pooled analysis of two phase 3 clinical trials of dalfampridine in patients with multiple sclerosis. Int J MS Care. 2014;16(3):153–60.
Limone BL, Sidovar MF, Coleman CI. Estimation of the effect of dalfampridine-ER on health utility by mapping the MSWS-12 to the EQ-5D in multiple sclerosis patients. Health Qual Life Outcomes. 2013;11:105.
Macdonell R, Nagels G, Laplaud DA, et al. Improved patient-reported health impact of multiple sclerosis: the ENABLE study of PR-fampridine. Mult Scler. 2016;22(7):944–54.
Jensen H, Ravnborg M, Mamoei S, et al. Changes in cognition, arm function and lower body function after slow-release fampridine treatment. Mult Scler. 2014;20(14):1872–80.
Jensen HB, Nielsen JL, Ravnborg M, et al. Effect of slow release-fampridine on muscle strength, rate of force development, functional capacity and cognitive function in an enriched population of MS patients. A randomized, double blind, placebo controlled study. Mult Scler Relat Disord. 2016;10:137–44.
Yapundich R, Applebee A, Bethoux F, et al. Evaluation of dalfampridine extended release 5 and 10 mg in multiple sclerosis: a randomized controlled trial. Int J MS Care. 2015;17(3):138–45.
Applebee A, Goodman AD, Mayadev AS, et al. Effects of dalfampridine extended-release tablets on 6-minute walk distance in patients with multiple sclerosis: a post hoc analysis of a double-blind, placebo-controlled trial. Clin Ther. 2015;37(12):2780–7.
Schmidt S, Oschmann P, Neau JP, et al. Walking, quality of life, and safety with prolonged-release fampridine treatment in clinical practice: interim results of the liberate study [abstract no. 51]. J Neurol Sci. 2015;357(Suppl 1):e21.
Allart E, Benoit A, Blanchard-Dauphin A, et al. Sustained-released fampridine in multiple sclerosis: effects on gait parameters, arm function, fatigue, and quality of life. J Neurol. 2015;262(8):1936–45.
Costa-Arpin E, Pato A, Rodríguez-Regal A, et al. Clinical response and tolerability of fampridine in clinical practice. Neurodegener Dis Manag. 2016;6(2):99–105.
US National Institutes of Health. ClinicalTrials.gov. 2017. https://www.clinicaltrials.gov. Accessed 10 July 2017.
Ben-Zacharia AB, Mathewson G. Symptom management in multiple sclerosis. In: Miller A, editor. Handbook of relapsing-remitting multiple sclerosis. Cham: Springer International Publishing; 2017. p. 115–34.
Harel A, Katz-Sand I. Treatment strategies in multiple sclerosis. In: Miller A, editor. Handbook of relapsing-remitting multiple sclerosis. Cham: Springer International Publishing; 2017. p. 67–97.
Larocca NG. Impact of walking impairment in multiple sclerosis: perspectives of patients and care partners. Patient. 2011;4(3):189–201.
Heesen C, Böhm J, Reich C, et al. Patient perception of bodily functions in multiple sclerosis: gait and visual function are the most valuable. Mult Scler. 2008;14(7):988–91.
Acknowledgements
During the peer review process, the manufacturer of fampridine PR was offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The preparation of this review was not supported by any external funding.
Conflict of interest
Esther Kim is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflict of interest.
Additional information about this Adis Drug Review can be found at http://www.medengine.com/Redeem/6128F06037461F38.
Additional information
The manuscript was reviewed by: G. Comi, Department of Neurology, University Vita-Salute San Raffaele, Scientific Institute San Raffaele, Milan, Italy; M. Linnebank, University Witten/Herdecke, Witten, Germany, Helios Klinik Hagen Ambrock, Hagen, Germany; T. Menge, Department of Neurology, Heinrich-Heine-University Düsseldorf, Düsseldorf, Germany.
Rights and permissions
About this article
Cite this article
Kim, E.S. Fampridine Prolonged Release: A Review in Multiple Sclerosis Patients with Walking Disability. Drugs 77, 1593–1602 (2017). https://doi.org/10.1007/s40265-017-0808-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-017-0808-z