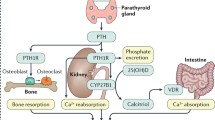
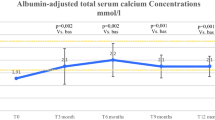
Abstract
Full-length recombinant human parathyroid hormone [rhPTH (1–84); Natpara®] is approved in the USA as an adjunct to calcium and vitamin D therapy for control of hypocalcaemia in patients with hypoparathyroidism. This article reviews the clinical efficacy and tolerability of rhPTH (1–84) in hypoparathyroidism and summarizes its pharmacological properties. In a pivotal phase III trial, subcutaneous rhPTH (1–84) was effective in maintaining albumin-corrected total serum calcium levels while reducing/eliminating the need for oral calcium and active vitamin D. rhPTH (1–84) had a generally acceptable tolerability profile in this trial, with <3 % of patients discontinuing treatment because of adverse events. Commonly occurring adverse reactions included hypocalcaemia, hypercalcaemia and hypercalciuria. As the first PTH replacement therapy for hypoparathyroid patients with hypocalcaemia, rhPTH (1–84) is an effective regimen, has generally acceptable tolerability and represents an important advance for the management of hypoparathyroidism.

Similar content being viewed by others
References
Isaia G, Marchetti M. PTH and PTH-related peptides. In: Brandi ML, Brown EM, editors. Hypoparathyroidism. Milan: Springer; 2015. p. 19–24.
Bilezikian JP, Khan A, Potts JT Jr, et al. Hypoparathyroidism in the adult: epidemiology, diagnosis, pathophysiology, target-organ involvement, treatment, and challenges for future research. J Bone Miner Res. 2011;26(10):2317–37.
Pajevic PD, Wein MN, Kronenberg HM. Parathyroid hormone actions on bone and kidney. In: Brandi ML, Brown EM, editors. Hypoparathyroidism. Milan: Springer; 2015. p. 99–109.
Shoback D. Clinical practice. Hypoparathyroidism. N Engl J Med. 2008;359(4):391–403.
Rubin MR, Bilezikian JP. Hypoparathyroidism: clinical features, skeletal microstructure and parathyroid hormone replacement. Arq Bras Endocrinol Metabol. 2010;54(2):220–6.
Powers J, Joy K, Ruscio A, et al. Prevalence and incidence of hypoparathyroidism in the United States using a large claims database. J Bone Miner Res. 2013;28(12):2570–6.
Rubin MR, Dempster DW, Zhou H, et al. Dynamic and structural properties of the skeleton in hypoparathyroidism. J Bone Miner Res. 2008;23(12):2018–24.
Clarke BL. Epidemiology of hypoparathyroidism. In: Brandi ML, Brown EM, editors. hypoparathyroidism. Milan: Springer; 2015. p. 139–54.
Wheeler AL, Shoback DM. Clinical presentation of hypoparathyroidism. In: Brandi ML, Brown EM, editors. Hypoparathyroidism. Milan: Springer; 2015. p. 155–65.
Horwitz MJ, Stewart AF. Hypoparathyroidism: is it time for replacement therapy? J Clin Endocrinol Metab. 2008;93(9):3307–9.
Egbuna OI, Brown EM. Hypoparathyroidism. In: Eisenbarth GS, editor. Immunoendocrinology: scientific and clinical aspects. New York: Humana Press; 2011. p. 501–17.
US FDA. FDA briefing document for the September 12, 2014 meeting of the Endocrinologic and Metabolic Drugs Advisory Committee. 2014. http://www.fda.gov/. Accessed 22 June 2015.
Arlt W, Fremerey C, Callies F, et al. Well-being, mood and calcium homeostasis in patients with hypoparathyroidism receiving standard treatment with calcium and vitamin D. Eur J Endocrinol. 2002;146(2):215–22.
US FDA. Natpara® (parathyroid hormone) for injection: US prescribing information. 2015. http://www.fda.gov/. Accessed 22 June 2015.
D’Amour P. Circulating PTH molecular forms: what we know and what we don’t. Kidney Int Suppl. 2006;70(Suppl 102s):S29–33.
Goltzman D, Karaplis AC. The PTH/vitaminD/FGF23 axis. In: Brandi ML, Brown EM, editors. Hypoparathyroidism. Milan: Springer; 2015. p. 69–79.
Clarke BL, Kay Berg J, Fox J, et al. Pharmacokinetics and pharmacodynamics of subcutaneous recombinant parathyroid hormone (1–84) in patients with hypoparathyroidism: an open-label, single-dose, phase I study. Clin Ther. 2014;36(5):722–36.
Sikjaer T, Amstrup AK, Rolighed L, et al. PTH(1–84) replacement therapy in hypoparathyroidism: a randomized controlled trial on pharmacokinetic and dynamic effects after 6 months of treatment. J Bone Miner Res. 2013;28(10):2232–43.
Sikjaer T, Rejnmark L, Rolighed L, et al. The effect of adding PTH(1–84) to conventional treatment of hypoparathyroidism: a randomized, placebo-controlled study. J Bone Miner Res. 2011;26(10):2358–70.
Sikjaer T, Rejnmark L, Thomsen JS, et al. Changes in 3-dimensional bone structure indices in hypoparathyroid patients treated with PTH(1–84): a randomized controlled study. J Bone Miner Res. 2012;27(4):781–8.
Rubin MR, Dempster DW, Sliney J Jr, et al. PTH(1–84) administration reverses abnormal bone-remodeling dynamics and structure in hypoparathyroidism. J Bone Miner Res. 2011;26(11):2727–36.
Rubin MR, Manavalan JS, Dempster DW, et al. Parathyroid hormone stimulates circulating osteogenic cells in hypoparathyroidism. J Clin Endocrinol Metab. 2011;96(1):176–86.
US FDA. Natpara (parathyroid hormone) clinical pharmacology biopharmaceutics review. 2015. http://www.fda.gov/. Accessed 22 June 2015.
Friedman PA, Goodman WG. PTH(1–84)/PTH(7-84): a balance of power. Am J Physiol Renal Physiol. 2006;290(5):F975–84.
Santini SA, Carrozza C, Vulpio C, et al. Assessment of parathyroid function in clinical practice: which parathyroid hormone assay is better? Clin Chem. 2004;50(7):1247–50.
D’Amour P. PTH assays and their clinical significance. In: Brandi ML, Brown EM, editors. Hypoparathyroidism. Milan: Springer; 2015. p. 25–32.
Mannstadt M, Clarke BL, Vokes T, et al. Efficacy and safety of recombinant human parathyroid hormone (1–84) in hypoparathyroidism (REPLACE): a double-blind, placebo-controlled, randomised, phase 3 study. Lancet Diabetes Endocrinol. 2013;1(4):275–83.
Cusano NE, Rubin MR, McMahon DJ, et al. PTH(1–84) is associated with improved quality of life in hypoparathyroidism through 5 years of therapy. J Clin Endocrinol Metab. 2014;99(10):3694–9.
Cusano NE, Rubin MR, McMahon DJ, et al. The effect of PTH(1–84) on quality of life in hypoparathyroidism. J Clin Endocrinol Metab. 2013;98(6):2356–61.
Cusano NE, Rubin MR, McMahon DJ, et al. Therapy of hypoparathyroidism with PTH(1–84): a prospective four-year investigation of efficacy and safety. J Clin Endocrinol Metab. 2013;98(1):137–44.
Rubin MR, Sliney J Jr, McMahon DJ, et al. Therapy of hypoparathyroidism with intact parathyroid hormone. Osteoporos Int. 2010;21(11):1927–34.
US FDA. Natpara (parathyroid hormone) medical review. 2015. http://www.fda.gov/. Accessed 22 June 2015.
Clarke BL, Mannstadt M, Vokes TJ, et al. Three-year safety and efficacy data for recombinant human parathyroid hormone, rhPTH(1–84), in the treatment of adults with hypoparathyroidism: the RACE study [abstract no. OC3.5]. In: 17th European Congress of Endocrinology; 2015.
Levine MA, Bilezikian JP, Clarke BL, et al. Long-term effects of recombinant human parathyroid hormone, rhPTH(1–84), on bone remodelling in patients with hypoparathyroidism: 3-year data from the open-label RACE study [abstract no. P82 plus poster]. In: 4th joint meeting of ECTS and IBMS; 2015.
Lakatos P, Bajnok L, Lagast H, et al. The REPEAT Study: an open-label clinical trial evaluating the safety and efficacy of recombinant human parathyroid hormone, rhPTH (1–84), for the treatment of hypoparathyroidism in Hungary [abstract no. OC6.4]. In: 16th European Congress of Endocrinology; 2014.
Sikjaer T, Rolighed L, Hess A, et al. Effects of PTH(1–84) therapy on muscle function and quality of life in hypoparathyroidism: results from a randomized controlled trial. Osteoporos Int. 2014;25(6):1717–26.
NPS Pharma. FDA approves Natpara® (parathyroid hormone) for injection as an adjunct to calcium and vitamin D to control hypocalcemia in patients with hypoparathyroidism. 2015. http://ir.npsp.com/releasedetail.cfm?releaseid=892722. Accessed 22 June 2015.
European Medicines Agency. Public summary of opinion on orphan designation—recombinant human parathyroid hormone for the treatment of hypoparathyroidism. 2014. http://www.ema.europa.eu. Accessed 22 June 2015.
NPS Pharma. European Medicines Agency validates marketing authorization application for Natpar® (parathyroid hormone (rDNA)) in hypoparathyroidism. 2014. http://ir.npsp.com/releasedetail.cfm?releaseid=885657. Accessed 22 June 2015.
Moen MD, Scott LJ. Recombinant full-length parathyroid hormone (1–84). Drugs. 2006;66(18):2371–81.
European Medicines Agency. Public statement on Preotact (PTH (parathyroid hormone)). 2014. http://www.ema.europa.eu/. Accessed 22 June 2015.
Cusano NE, Rubin MR, Irani D, et al. Use of parathyroid hormone in hypoparathyroidism. J Endocrinol Invest. 2013;36(11):1121–7.
Ramakrishnan Y, Cocks HC. Impact of recombinant PTH on management of hypoparathyroidism: a systematic review. Eur Arch Otorhinolaryngol. 2015;. doi:10.1007/s00405-014-3484-6.
Andrews EB, Gilsenan AW, Midkiff K, et al. The US postmarketing surveillance study of adult osteosarcoma and teriparatide: study design and findings from the first 7 years. J Bone Miner Res. 2012;27(12):2429–37.
Cipriani C, Irani D, Bilezikian JP. Safety of osteoanabolic therapy: a decade of experience. J Bone Miner Res. 2012;27(12):2419–28.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made by the authors on the basis of scientific and editorial merit. Esther Kim and Gillian Keating are salaried employees of Adis/Springer.
Additional information
The manuscript was reviewed by: P. Divieti-Pajevic, Department of Molecular and Cell Biology, Goldman School of Dental Medicine, Boston University, Boston, MA, USA; T. Sikjaer, Department of Endocrinology and Internal Medicine, Aarhus University Hospital, Aarhus, Denmark; A. L. Wheeler, Department of Medicine, University of California, San Francisco, CA, USA.
Rights and permissions
About this article
Cite this article
Kim, E.S., Keating, G.M. Recombinant Human Parathyroid Hormone (1–84): A Review in Hypoparathyroidism. Drugs 75, 1293–1303 (2015). https://doi.org/10.1007/s40265-015-0438-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-015-0438-2