Abstract
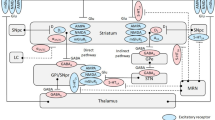
The pathological processes underlying Parkinson’s disease (PD) involve more than dopamine cell loss within the midbrain. These non-dopaminergic neurotransmitters include noradrenergic, serotonergic, glutamatergic, and cholinergic systems within cortical, brainstem and basal ganglia regions. Several non-dopaminergic treatments are now in clinical use to treat motor symptoms of PD, or are being evaluated as potential therapies. Agents for symptomatic monotherapy and as adjunct to dopaminergic therapies for motor symptoms include adenosine A2A antagonists and the mixed monoamine-B inhibitor (MAO-BI) and glutamate release agent safinamide. The largest area of potential use for non-dopaminergic drugs is as add-on therapy for motor fluctuations. Thus adenosine A2A antagonists, safinamide, and the antiepileptic agent zonisamide can extend the duration of action of levodopa. To reduce levodopa-induced dyskinesia, drugs that target overactive glutamatergic neurotransmission can be used, and include the non-selective N-methyl d-aspartate antagonist amantadine. More recently, selective metabotropic glutamate receptor (mGluR5) antagonists are being evaluated in phase II randomized controlled trials. Serotonergic agents acting as 5-HT2A/2C antagonists, such as the atypical antipsychotic clozapine, may also reduce dyskinesia. 5-HT1A agonists theoretically can reduce dyskinesia, but in practice, may also worsen PD motor symptoms, and so clinical applicability has not yet been shown. Noradrenergic α2A antagonism using fipamezole can potentially reduce dyskinesia. Several non-dopaminergic agents have also been investigated to reduce non-levodopa-responsive motor symptoms such as gait and tremor. Thus the cholinesterase inhibitor donepezil showed mild benefit in gait, while the predominantly noradrenergic re-uptake inhibitor methylphenidate had conflicting results in advanced PD subjects. Tremor in PD may respond to muscarinic M4 cholinergic antagonists (anticholinergics), but tolerability is often poor. Alternatives include β-adrenergic antagonists such as propranolol. Other options include 5-HT2A antagonists, and drugs that have mixed binding properties involving serotonin and acetylcholine, such as clozapine and the antidepressant mirtazapine, can be effective in reducing PD tremor. Many other non-dopaminergic agents are in preclinical and phase I/II early stages of study, and the reader is directed to recent reviews. While levodopa remains the most effective agent to treat motor symptoms in PD, the overall approach to using non-dopaminergic drugs in PD is to reduce reliance on levodopa and to target non-levodopa-responsive symptoms.
Similar content being viewed by others
References
Halliday GM, Li YW, Blumbergs PC, et al. Neuropathology of immunohistochemically identified brainstem neurons in Parkinson’s disease. Ann Neurol. 1990;27:373–85.
Zarow C, Lyness SA, Mortimer JA, et al. Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol. 2003;60:337–41.
Braak H, Del Tredici K, Rüb U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211.
Halliday GM, Blumbergs PC, Cotton RG, et al. Loss of brainstem serotonin- and substance P-containing neurons in Parkinson’s disease. Brain Res. 1990;510(1):104–7.
Paulus W, Jellinger K. The neuropathologic basis of different clinical subgroups of Parkinson’s disease. J Neuropathol Exp Neurol. 1991;50:743–55.
Kish SJ, Tong J, Hornykiewicz O, et al. Preferential loss of serotonin markers in caudate versus putamen in Parkinson’s disease. Brain. 2008;131:120–31.
Lang AE, Obeso JA. Time to move beyond nigrostriatal dopamine deficiency in Parkinson’s disease. Ann Neurol. 2004;55:761–5.
Huot P, Johnston TH, Koprich JB, et al. The pharmacology of l-DOPA-induced dyskinesia in Parkinson’s disease. Pharmacol Rev. 2013;65:1–52.
Bezard E, Brotchie JM, Gross CE. Pathophysiology of levodopa-induced dyskinesia: potential for new therapies. Nat Rev Neurosci. 2001;2:577–88.
Brotchie JM. Nondopaminergic mechanisms in levodopa-induced dyskinesia. Mov Disord. 2005;20:919–31.
Huot P, Fox SH. Nondopaminergic treatments for Parkinson’s disease. Neurodegener Dis Manag. 2011;6:491–512.
Kalia L, Brotchie JM, Fox SH. Non dopaminergic therapies for PD motor symptoms; update on recent clinical trials. Mov Disord. 2013;28(2):131–44.
Gomez-Mancilla B, Bedard PJ. Effect of nondopaminergic drugs on l-dopa-induced dyskinesias in MPTP-treated monkeys. Clin Neuropharmacol. 1993;16:418–27.
Fox SH, Brotchie JM, Lang AE. Non-dopaminergic treatments in development for Parkinson’s disease. Lancet Neurol. 2008;7:927–38.
Schwarzschild MA, Agnati L, Fuxe K, et al. Targeting adenosine A2A receptors in Parkinson’s disease. Trends Neurosci. 2006;29:647–54.
Nash JE, Brotchie JM. A common signaling pathway for striatal NMDA and adenosine A2a receptors: implications for the treatment of Parkinson’s disease. J Neurosci. 2000;20:7782–9.
Kanda T, Jackson MJ, Smith LA, et al. Adenosine A2A antagonist: a novel antiparkinsonian agent that does not provoke dyskinesia in parkinsonian monkeys. Ann Neurol. 1998;43:507–13.
Fernandez HH, Greeley DR, Zweig RM, et al. Istradefylline as monotherapy for Parkinson disease: results of the 6002-US-051 trial. Parkinsonism Relat Disord. 2010;16:16–20.
Merck. A phase 3, double-blind, double-dummy, placebo- and active-controlled dose-range-finding efficacy and safety study of preladenant in subjects with early Parkinson’s disease. ClinicalTrials.gov (Internet). Bethesda: National Library of Medicine (US); 2000. http://clinicaltrials.gov/show/NCT01155479. Cited 13 May 2013.
Stocchi F, Borgohain R, Onofrj M, et al. A randomized, double-blind, placebo-controlled trial of safinamide as add-on therapy in early Parkinson’s disease patients. Mov Disord. 2012;27(1):106–12.
Barone P, Fernandez H, Ferreira J, et al. Safinamide as an add-on therapy to a stable dose of a single dopamine agonist: results from a randomized, placebo-controlled, 24-week multicenter trial in early idiopathic Parkinson disease (PD) patients (MOTION Study). In: Abstract 65th Annual American Academy of Neurology poster 1.061; 2013.
Schapira AH, Fox S, Hauser R, et al., on behalf of the SETTLE Investigators. Safinamide add on to l-dopa: a randomized, placebo-controlled, 24-week global trial in patients with Parkinson’s disease (PD) and motor fluctuations (SETTLE). In: Abstract 65th Annual American Academy of Neurology poster 1.062; 2013.
Murata M, Hasegawa K, Kanazawa I, Japan Zonisamide on PD Study Group. Zonisamide improves motor function in Parkinson disease: a randomized, double-blind study. Neurology. 2007;68(1):45–50.
Bara-Jimenez W, Sherzai A, Dimitrova T, et al. Adenosine A(2A) receptor antagonist treatment of Parkinson’s disease. Neurology. 2003;61(3):293–6.
Hauser RA, Hubble JP, Truong DD. Randomized trial of the adenosine A(2A) receptor antagonist istradefylline in advanced PD. Neurology. 2003;61(3):297–303.
Stacy M, Silver D, Mendis T, et al. A 12-week, placebo-controlled study (6002-US-006) of istradefylline in Parkinson disease. Neurology. 2008;70(23):2233–40.
LeWitt PA, Guttman M, Tetrud JW, et al. Adenosine A2A receptor antagonist istradefylline (KW-6002) reduces “off” time in Parkinson’s disease: a double-blind, randomized, multicenter clinical trial (6002-US-005). Ann Neurol. 2008;63(3):295–302.
Mizuno Y, Hasegawa K, Kondo T, et al. Clinical efficacy of istradefylline (KW-6002) in Parkinson’s disease: a randomized, controlled study. Mov Disord. 2010;25(10):1437–43.
Hauser RA, Shulman LM, Trugman JM, et al. Study of istradefylline in patients with Parkinson’s disease on levodopa with motor fluctuations. Mov Disord. 2008;23(15):2177–85.
Mizuno Y, Kondo T, the Japanese Istradefylline Study Group. Adenosine A2A receptor antagonist istradefylline reduces daily off time in Parkinson’s disease. Mov Disord. 2013. doi:10.1002/mds.25418.
Hauser RA, Cantillon M, Pourcher E, et al. Preladenant in patients with Parkinson’s disease and motor fluctuations: a phase 2, double-blind, randomised trial. Lancet Neurol. 2011;10(3):221–9.
Merck. A phase 3, 12-week, double-blind, double-dummy, placebo- and active-controlled efficacy and safety study of preladenant in subjects with moderate to severe Parkinson’s disease. ClinicalTrials.gov (Internet). Bethesda: National Library of Medicine (US); 2000. http://clinicaltrials.gov/show/NCT01155466. Cited 13 May 2013.
Biotie. A double-blind, randomized, placebo-controlled study of the safety and efficacy of SYN115 as adjunctive therapy in levodopa-treated Parkinson’s subjects with end of dose wearing off. ClinicalTrials.gov (Internet). Bethesda: National Library of Medicine (US); 2000. http://clinicaltrials.gov/show/NCT01283594. Cited 23 May 2013.
Fox SH, Lang AE, Brotchie JM. Translation of non-dopaminergic treatments for levodopa-induced dyskinesia from MPTP-lesioned nonhuman primates to phase IIa clinical studies: keys to success and roads to failure. Mov Disord. 2006;21:1578–94.
Calabresi P, Giacomini P, Centonze D, et al. Levodopa-induced dyskinesia: a pathological form of striatal synaptic plasticity? Ann Neurol. 2000;47:60–8.
Conn PJ, Battaglia G, Marino MJ, et al. Metabotropic glutamate receptors in the basal ganglia motor circuit. Nat Rev Neurosci. 2005;6(10):787–98.
Duty S. Targeting glutamate receptors to tackle the pathogenesis, clinical symptoms and levodopa-induced dyskinesia associated with Parkinson’s disease. CNS Drugs. 2012;26(12):1017–32.
Fox SH, Katzenschlager R, Lim SY, et al. Movement disorder society evidence-based medicine review update: treatments for the motor symptoms of Parkinson’s disease. Mov Disord. 2011;26(Suppl 3):S2–41.
Wolf E, Seppi K, Katzenschlager R, et al. Long-term antidyskinetic efficacy of amantadine in Parkinson’s disease. Mov Disord. 2010;25:1357–63.
Adamas Pharmaceuticals, Inc. Extended Release Amantadine Safety and Efficacy Study in Levodopa-Induced Dyskinesia (EASED Study). ClinicalTrials.gov (Internet). Bethesda: National Library of Medicine (US); 2000. http://clinicaltrials.gov/show/NCT01397422. Cited 9 May 2013.
Parkinson Study Group. Evaluation of dyskinesias in a pilot, randomized, placebo-controlled trial of remacemide in advanced Parkinson disease. Arch Neurol. 2001;58:1660–8.
Lees A, Fahn S, Eggert KM, et al. Perampanel, an AMPA antagonist, found to have no benefit in reducing “off” time in Parkinson’s disease. Mov Disord. 2012;27(2):284–8.
Johnston TH, Brotchie JM. Drugs in development for Parkinson’s disease. Curr Opin Investig Drugs. 2004;5(7):720–6.
Rascol O, Fox SH, Gasparini F, et al. Use of metabotropic glutamate 5 receptor antagonists for treatment of levodopa-induced dyskinesias. Mov Disord (in press).
Berg D, Godau J, Trenkwalder C, et al. AFQ056 treatment of levodopa-induced dyskinesias: results of 2 randomized controlled trials. Mov Disord. 2011;26(7):1243–50.
Tison F, Durif F, Ferrand C, et al. Safety, tolerability and anti-dyskinetic efficacy of dipraglurant, a novel mGluR5 negative allosteric modulator (NAM) in Parkinson’s disease (PD) patients with levodopa-induced dyskinesia (LID). In: Abstract 65th Annual American Academy of Neurology poster 23.004; Mar 2013.
Mavridis M, Degryse AD, Lategan AJ, et al. Effects of locus coeruleus lesions on parkinsonian signs, striatal dopamine and substantia nigra cell loss after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in monkeys: a possible role for the locus coeruleus in the progression of Parkinson’s disease. Neuroscience. 1991;41(2–3):507–23.
Iravani MM, Jenner P. Mechanisms underlying the onset and expression of levodopa-induced dyskinesia and their pharmacological manipulation. J Neural Transm. 2011;118(12):1661–90.
Henry B, Fox SH, Peggs D, et al. The alpha2-adrenergic receptor antagonist idazoxan reduces dyskinesia and enhances anti-parkinsonian actions of l-dopa in the MPTP-lesioned primate model of Parkinson’s disease. Mov Disord. 1999;14:744–53.
Barnum CJ, Bhide N, Lindenbach D, et al. Effects of noradrenergic denervation on l-DOPA-induced dyskinesia and its treatment by α- and β-adrenergic receptor antagonists in hemiparkinsonian rats. Pharmacol Biochem Behav. 2012;100(3):607–15.
Lewitt PA, Hauser RA, Lu M, et al. Randomized clinical trial of fipamezole for dyskinesia in Parkinson disease (FJORD study). Neurology. 2012;79(2):163–9.
Carta M, Carlsson T, Kirik D, Bjorklund A. Dopamine released from 5-HT terminals is the cause of l-DOPA-induced dyskinesia in parkinsonian rats. Brain. 2007;130:1819–33.
Munoz A, Li Q, Gardoni F, et al. Combined 5-HT1A and 5-HT1B receptor agonists for the treatment of l-DOPA-induced dyskinesia. Brain. 2008;131:3380–94.
Kleedorfer B, Lees AJ, Stern GM. Buspirone in the treatment of levodopa induced dyskinesias. J Neurol Neurosurg Psychiatry. 1991;54:376–7.
Goetz CG, Damier P, Hicking C, et al. Sarizotan as a treatment for dyskinesias in Parkinson’s disease: a double-blind placebo-controlled trial. Mov Disord. 2007;22:179–86.
Goetz CG, Laska E, Hicking C, et al. Placebo influences on dyskinesia in Parkinson’s disease. Mov Disord. 2008;23:700–7.
Sage JJ, Hauser RA, Cordon ME, Gonzalez MA, Tani Y, Koyamam M, Apfel SC, Reed RF, Okamato M, Gertbner JM. Pilot study of the efficacy and safety of piclozotan in Parkinson’s disease patients with l-dopa induced motor complications. Mov Disord. 2009;24(Suppl 1):S277.
Rascol O, Bronzova J, Hauser RA, et al. Pardoprunox as adjunct therapy to levodopa in patients with Parkinson’s disease experiencing motor fluctuations: results of a double-blind, randomized, placebo-controlled, trial. Parkinsonism Relat Disord. 2012;18(4):370–6.
Kapur S, Seeman P. Does fast dissociation from the dopamine d(2) receptor explain the action of atypical antipsychotics? A new hypothesis. Am J Psychiatry. 2001;158(3):360–9.
Durif F, Debilly B, Galitzky M, et al. Clozapine improves dyskinesias in Parkinson disease: a double-blind, placebo-controlled study. Neurology. 2004;62(3):381–8.
Katzenschlager R, Manson AJ, Evans A, et al. Low dose quetiapine for drug induced dyskinesias in Parkinson’s disease: a double blind cross over study. J Neurol Neurosurg Psychiatry. 2004;75(2):295–7.
Hill MP, Bezard E, McGuire SG, et al. Novel antiepileptic drug levetiracetam decreases dyskinesia elicited by l-dopa and ropinirole in the MPTP-lesioned marmoset. Mov Disord. 2003;18(11):1301–5.
Bezard E, Hill MP, Crossman AR, et al. Levetiracetam improves choreic levodopa-induced dyskinesia in the MPTP-treated macaque. Eur J Pharmacol. 2004;485(1–3):159–64.
Hill MP, Ravenscroft P, Bezard E, et al. Levetiracetam potentiates the antidyskinetic action of amantadine in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned primate model of Parkinson’s disease. J Pharmacol Exp Ther. 2004;310(1):386–94.
Wolz M, Löhle M, Strecker K, et al. Levetiracetam for levodopa-induced dyskinesia in Parkinson’s disease: a randomized, double-blind, placebo-controlled trial. J Neural Transm. 2010;117(11):1279–86.
Stathis P, Konitsiotis S, Tagaris G, et al. Levetiracetam for the management of levodopa-induced dyskinesias in Parkinson’s disease. Mov Disord. 2011;26(2):264–70.
Wong KK, Alty JE, Goy AG, et al. A randomized, double-blind, placebo-controlled trial of levetiracetam for dyskinesia in Parkinson’s disease. Mov Disord. 2011;26(8):1552–5.
Lyons KE, Pahwa R. Efficacy and tolerability of levetiracetam in Parkinson disease patients with levodopa-induced dyskinesia. Clin Neuropharmacol. 2006;29(3):148–53.
Zesiewicz TA, Sullivan KL, Maldonado JL, et al. Open-label pilot study of levetiracetam (Keppra) for the treatment of levodopa-induced dyskinesias in Parkinson’s disease. Mov Disord. 2005;20(9):1205–9.
Hely MA, Reid WG, Adena MA, et al. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837–44.
Balaban CD. Neural substrates linking balance control and anxiety. Physiol Behav. 2002;77:469–75.
Tohgi H, Abe T, Takahashi S. The effects of l-threo-3,4-dihydroxyphenylserine on the total norepinephrine and dopamine concentrations in the cerebrospinal fluid and freezing gait in parkinsonian patients. J Neural Transm Park Dis Dement Sect. 1993;5:27–34.
Fukada K, Endo T, Yokoe M, et al. l-Threo-3,4-dihydroxyphenylserine (l-DOPS) co-administered with entacapone improves freezing of gait in Parkinson’s disease. Med Hypotheses. 2013;80(2):209–12.
Devilbiss DM, Berridge CW. Low-dose methylphenidate actions on tonic and phasic locus coeruleus discharge. J Pharmacol Exp Ther. 2006;319:1327–35.
Volkow ND, Wang G, Fowler JS, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci. 2001;21(2):RC121.
Espay AJ, Dwivedi AK, Payne M, et al. Methylphenidate for gait impairment in Parkinson disease: a randomized clinical trial. Neurology. 2011;76(14):1256–62.
Moreau C, Delval A, Defebvre L, et al. Methylphenidate for gait hypokinesia and freezing in patients with Parkinson’s disease undergoing subthalamic stimulation: a multicentre, parallel, randomised, placebo-controlled trial. Lancet Neurol. 2012;11(7):589–96.
Bohnen NI, Müller ML, Koeppe RA, et al. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology. 2009;73(20):1670–6.
Karachi C, Grabli D, Bernard FA, et al. Cholinergic mesencephalic neurons are involved in gait and postural disorders in Parkinson disease. J Clin Investig. 2010;120(8):2745–54.
Chung KA, Lobb BM, Nutt JG, et al. Effects of a central cholinesterase inhibitor on reducing falls in Parkinson disease. Neurology. 2010;75:1263–9.
Hall D, et al. Varenicline for the treatment of postural and gait dysfunction in Parkinson disease. ClinicalTrials.gov (Internet). Bethesda: National Library of Medicine (US); 2000. http://clinicaltrials.gov/show/NCT01341080. Cited 14 May 2013.
DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13(7):281–5.
Ghaemi M, Raethjen J, Hilker R, et al. Monosymptomatic resting tremor and Parkinson’s disease: a multitracer positron emission tomographic study. Mov Disord. 2002;17(4):782–8.
Asenbaum S, Pirker W, Angelberger P, et al. [123I]beta-CIT and SPECT in essential tremor and Parkinson’s disease. J Neural Transm. 1998;105(10–12):1213–28.
Benamer HT, Oertel WH, Patterson J, et al. Prospective study of presynaptic dopaminergic imaging in patients with mild parkinsonism and tremor disorders: part 1. Baseline and 3-month observations. Mov Disord. 2003;18(9):977–84.
Pirker W. Correlation of dopamine transporter imaging with parkinsonian motor handicap: how close is it? Mov Disord. 2003;18(Suppl 7):S43–51.
Collins-Praino LE, Paul NE, Rychalsky KL, et al. Pharmacological and physiological characterization of the tremulous jaw movement model of parkinsonian tremor: potential insights into the pathophysiology of tremor. Front Syst Neurosci. 2011;5:49.
Doder M, Rabiner EA, Turjanski N, et al. Tremor in Parkinson’s disease and serotonergic dysfunction: an 11C-WAY 100635 PET study. Neurology. 2003;60(4):601–5.
Loane C, Wu K, Bain P, et al. Serotonergic loss in motor circuitries correlates with severity of action-postural tremor in PD. Neurology. 2013;80:1850–5.
Carlson BB, Wisniecki A, Salamone JD. Local injections of the 5-hydroxytryptamine antagonist mianserin into substantia nigra pars reticulata block tremulous jaw movements in rats: studies with a putative model of parkinsonian tremor. Psychopharmacology (Berl). 2003;165(3):229–37.
Friedman JH, Koller WC, Lannon MC, et al. Benztropine versus clozapine for the treatment of tremor in Parkinson’s disease. Neurology. 1997;48(4):1077–81.
Trosch RM, Friedman JH, Lannon MC, et al. Clozapine use in Parkinson’s disease: a retrospective analysis of a large multicentered clinical experience. Mov Disord. 1998;13(3):377–82.
Bonuccelli U, Ceravolo R, Salvetti S, et al. Clozapine in Parkinson’s disease tremor. Effects of acute and chronic administration. Neurology. 1997;49(6):1587–90.
Gordon PH, Pullman SL, Louis ED, et al. Mirtazapine in Parkinsonian tremor. Parkinsonism Relat Disord. 2002;9(2):125–6.
Crosby NJ, Deane KH, Clarke CE. Beta-blocker therapy for tremor in Parkinson’s disease. Cochrane Database Syst Rev. 2003;(1):CD003361.
Acknowledgments
No funding was provided for preparation of the paper and there are relevant conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fox, S.H. Non-dopaminergic Treatments for Motor Control in Parkinson’s Disease. Drugs 73, 1405–1415 (2013). https://doi.org/10.1007/s40265-013-0105-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-013-0105-4