Abstract
Introduction
Fluoroquinolones, clarithromycin, linezolid, tigecycline, cefditoren, doxycycline, and trimethoprim–sulfamethoxazole are known to be associated with hypoglycemia, but few studies have considered concomitant glucose-lowering medications.
Objective
The objective of this study was to evaluate the association between hypoglycemia and antibiotics using the US Food and Drug Administration Adverse Event Reporting System (FAERS), while accounting for concomitant glucose-lowering medications including sulfonylureas and meglitinides.
Methods
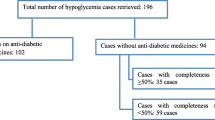
FAERS reports from 1 January 2004 to 31 December 2017 were included in the study. Reporting odds ratios (RORs) and corresponding 95% confidence intervals (CIs) for the association between antibiotics and hypoglycemia were calculated. An association was considered to be statistically significant when the lower limit of the 95% CI was > 1.0.
Results
A total of 2,334,959 reports (including 18,466 hypoglycemia reports) were considered, after inclusion criteria were applied. Statistically significant hypoglycemia RORs (95% CI) for antibiotics were: cefditoren 14.03 (8.93–22.03), tigecycline 3.32 (1.95–5.65), clarithromycin 2.41 (1.89–3.08), ertapenem 2.07 (1.14–3.75), moxifloxacin 2.06 (1.59–2.65), levofloxacin 1.66 (1.37–2.01), and linezolid 1.54 (1.07–2.20). After adjusting for concomitant sulfonylureas and meglitinides, the following antibiotics were still significantly associated with hypoglycemia: cefditoren 14.25 (9.08–22.39), tigecycline 3.34 (1.96–5.68), ertapenem 1.93 (1.03–3.60), and clarithromycin 1.56 (1.15–2.11).
Conclusion
In many patients, antibiotics, including fluoroquinolones, are associated with hypoglycemia when they are also taking sulfonylureas or meglitinides. Cefditoren, tigecycline, ertapenem, and clarithromycin are associated with hypoglycemia even if not taken with sulfonylureas or meglitinides. The association between ertapenem and hypoglycemia has not been previously reported.



Similar content being viewed by others
Data-sharing statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Food and Drug Administration. FDA updates warnings for fluoroquinolone antibiotics on risks of mental health and low blood sugar adverse reactions. https://www.fda.gov/news-events/press-announcements/fda-updates-warnings-fluoroquinolone-antibiotics-risks-mental-health-and-low-blood-sugar-adverse. Accessed 5 Nov 2019.
Frier BM. Hypoglycaemia in diabetes mellitus: epidemiology and clinical implications. Nat Rev Endocrinol. 2014;10:711–22.
Viswanathan P, Iarikov D, Wassel R, Davidson A, Nambiar S. Hypoglycemia in patients treated with linezolid. Clin Infect Dis. 2014;59:e93–5.
Kadoyama K, Sakaeda T, Tamon A, Okuno Y. Adverse event profile of tigecycline: data mining of the public version of the U.S. Food and Drug Administration Adverse Event Reporting System. Biol Pharm Bull. 2012;35:967–70.
Chou HW, Wang JL, Chang CH, Lee JJ, Shau WY, Lai MS. Risk of severe dysglycemia among diabetic patients receiving levofloxacin, ciprofloxacin, or moxifloxacin in Taiwan. Clin Infect Dis. 2013;57:971–80.
Mohr JF, McKinnon PS, Peymann PJ, Kenton I, Septimus E, Okhuysen PC. A retrospective, comparative evaluation of dysglycemias in hospitalized patients receiving gatifloxacin, levofloxacin, ciprofloxacin, or ceftriaxone. Pharmacotherapy. 2005;25:1303–9.
Eshraghian A, Omrani GR. Cotrimoxazole-induced hypoglycemia in outpatient setting. Nutrition. 2014;30:959.
Lee AJ, Maddix DS. Trimethoprim/sulfamethoxazole-induced hypoglycemia in a patient with acute renal failure. Ann Pharmacother. 1997;31:727–32.
Johnson JA, Kappel JE, Sharif MN. Hypoglycemia secondary to trimethoprim/sulfamethoxazole administration in a renal transplant patient. Ann Pharmacother. 1993;27:304–6.
Tan CH, Shelley C, Harman KE. Doxycycline-induced hypoglycaemia. Clin Exp Dermatol. 2016;41:43–4.
Odeh M, Oliven A. Doxycycline-induced hypoglycemia. J Clin Pharmacol. 2000;40:1173–4.
Basaria S, Braga M, Moore WT. Doxycycline-induced hypoglycemia in a nondiabetic young man. South Med J. 2002;95:1353–4.
Ito M, Fukuda M, Suzuki Y, Wakamoto H, Ishii E. Carnitine-related hypoglycemia caused by 3 days of pivalate antibiotic therapy in a patient with severe muscular dystrophy: a case report. BMC Pediatr. 2017;17:73.
Makino Y, Sugiura T, Ito T, Sugiyama N, Koyama N. Carnitine-associated encephalopathy caused by long-term treatment with an antibiotic containing pivalic acid. Pediatrics. 2007;120:e739–41.
Parekh TM, Raji M, Lin YL, Tan A, Kuo YF, Goodwin JS. Hypoglycemia after antimicrobial drug prescription for older patients using sulfonylureas. JAMA Intern Med. 2014;174:1605–12.
Khamaisi M, Leitersdorf E. Severe hypoglycemia from clarithromycin-repaglinide drug interaction. Pharmacotherapy. 2008;28:682–4.
Clarithromycin [package insert]. North Chicago: Abbott Laboratories; 2000. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/050662s044s050,50698s026s030,050775s015s019lbl.pdf. Accessed 5 Nov 2019.
Food and Drug Administration. FDA Adverse Event Reporting System (FAERS). https://www.fda.gov/drugs/surveillance/fda-adverse-event-reporting-system-faers. Accessed 5 Nov 2019.
Food and Drug Administration. Drugs@FDA: FDA-approved drugs. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm. Accessed 5 Nov 2019.
Evoy KE, Teng C, Encarnacion VG, et al. Comparison of quetiapine abuse and misuse reports to the FDA Adverse Event Reporting System with other second-generation antipsychotics. Subst Abuse. 2019;13:1–8.
Teng C, Reveles KR, Obodozie-Ofoegbu OO, Frei CR. Clostridium difficile infection risk with important antibiotic classes: an analysis of the FDA Adverse Event Reporting System. Int J Med Sci. 2019;16(5):630–5.
Teng C, Walter EA, Gaspar DK, Obodozie-Ofoegbu OO, Frei CR. Torsades de pointes and QT prolongation associations with antibiotics: a pharmacovigilance study of the FDA Adverse Event Reporting System. Int J Med Sci. 2019;16(7):1018–22.
Teng C, Baus C, Wilson JP, Frei CR. Rhabdomyolysis associations with antibiotics: a pharmacovigilance study of the FDA Adverse Event Reporting System (FAERS). Int J Med Sci. 2019;16(11):1504–9.
Patek TM, Teng C, Kennedy KE, Alvarez CA, Frei CR. Comparing acute kidney injury reports among antibiotics: a pharmacovigilance study of the FDA Adverse Event Reporting System (FAERS). Drug Saf. 2019. https://doi.org/10.1007/s40264-019-00873-8.
McConeghy KW, Soriano MM, Danziger LH. A quantitative analysis of FDA adverse event reports with oral bisphosphonates and Clostridium difficile. Pharmacotherapy. 2016;36:1095–101.
Evans SJ, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 2001;10:483–6.
MedDRA MSSO. Introductory guide for Standardised MedDRA Queries (SMQs) version 21.0. http://www.meddra.org/sites/default/files/guidance/file/smq_intguide_21_0_english.pdf. Accessed 5 Nov 2019.
Bate A, Evans SJ. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 2009;18:427–36.
Aspinall SL, Good CB, Jiang R, McCarren M, Dong D, Cunningham FE. Severe dysglycemia with the fluoroquinolones: a class effect? Clin Infect Dis. 2009;49:402–8.
Food and Drug Administration (9/9/2008). Determination that TEQUIN (gatifloxacin) was withdrawn from sale for reasons of safety or effectiveness. https://www.federalregister.gov/documents/2008/09/09/E8-20938/determination-that-tequin-gatifloxacin-was-withdrawn-from-sale-for-reasons-of-safety-or. Accessed 5 Nov 2019.
Bito M, Tomita T, Komori M, Taogoshi T, Kimura Y, Kihira K. The mechanisms of insulin secretion and calcium signaling in pancreatic beta-cells exposed to fluoroquinolones. Biol Pharm Bull. 2013;36:31–5.
Saraya A, Yokokura M, Gonoi T, Seino S. Effects of fluoroquinolones on insulin secretion and beta-cell ATP-sensitive K+ channels. Eur J Pharmacol. 2004;497:111–7.
Ashcroft FM. Mechanisms of the glycaemic effects of sulfonylureas. Horm Metab Res. 1996;28:456–63.
Niemi M, Neuvonen PJ, Kivisto KT. The cytochrome P4503A4 inhibitor clarithromycin increases the plasma concentrations and effects of repaglinide. Clin Pharmacol Ther. 2001;70:58–65.
Eberl S, Renner B, Neubert A, et al. Role of p-glycoprotein inhibition for drug interactions: evidence from in vitro and pharmacoepidemiological studies. Clin Pharmacokinet. 2007;46:1039–49.
Acknowledgements
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs, the National Institutes of Health, or the authors’ affiliated institutions.
Author information
Authors and Affiliations
Contributions
Study concept and design: CT and CRF. Statistical analysis: CT. Interpretation of data: all authors. Drafting of the manuscript: KEK, CRF, and CT. Critical revision of the manuscript for important intellectual content: all authors. Study supervision: CRF.
Corresponding author
Ethics declarations
Funding
No funding was sought for this research study. Dr. Frei was supported, in part, by a National Institutes of Health (NIH) Clinical and Translational Science Award (National Center for Advancing Translational Sciences, UL1 TR001120, UL1 TR002645, and TL1 TR002647) while the study was being conducted. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Conflict of interest
Kaitlin E. Kennedy, Chengwen Teng, Taylor M. Patek, and Christopher R. Frei have no conflicts of interest that are directly relevant to the content of this study.
Rights and permissions
About this article
Cite this article
Kennedy, K.E., Teng, C., Patek, T.M. et al. Hypoglycemia Associated with Antibiotics Alone and in Combination with Sulfonylureas and Meglitinides: An Epidemiologic Surveillance Study of the FDA Adverse Event Reporting System (FAERS). Drug Saf 43, 363–369 (2020). https://doi.org/10.1007/s40264-019-00901-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-019-00901-7