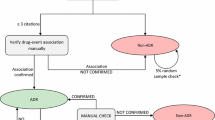
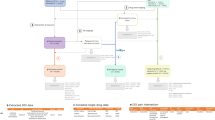
Abstract
The safety profile of a drug evolves over its lifetime on the market; there are bound to be changes in the circumstances of a drug’s clinical use which may give rise to previously unobserved adverse effects, hence necessitating surveillance postmarketing. Postmarketing surveillance has traditionally been carried out by systematic manual review of spontaneous reports of adverse drug reactions. Vast improvements in computing capabilities have provided opportunities to automate signal detection, and several worldwide initiatives are exploring new approaches to facilitate earlier detection, primarily through mining of routinely-collected data from electronic healthcare records (EHR). This paper provides an overview of ongoing initiatives exploring data from EHR for signal detection vis-à-vis established spontaneous reporting systems (SRS). We describe the role SRS has played in regulatory decision making with respect to safety issues, and evaluate the potential added value of EHR-based signal detection systems to the current practice of drug surveillance. Safety signal detection is both an iterative and dynamic process. It is in the best interest of public health to integrate and understand evidence from all possibly relevant information sources on drug safety. Proper evaluation and communication of potential signals identified remains an imperative and should accompany any signal detection activity.

Similar content being viewed by others
References
Zarin DA, Young JL, West JC. Challenges to evidence-based medicine: a comparison of patients and treatments in randomized controlled trials with patients and treatments in a practice research network. Soc Psychiatry Psychiatr Epidemiol. 2005;40(1):27–35.
Heiat A, Gross CP, Krumholz HM. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch Intern Med. 2002;162(15):1682–8.
Black N. Why we need observational studies to evaluate the effectiveness of health care. BMJ. 1996;312(7040):1215–8.
Papanikolaou PN, Christidi GD, Ioannidis JP. Comparison of evidence on harms of medical interventions in randomized and nonrandomized studies. CMAJ. 2006;174(5):635–41.
US FDA. FDA issues public health warning on phenylpropanolamine. Available from URL: http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm150763.htm. Accessed 9 Jan 2013.
US FDA. FDA requires additional labeling for over-the-counter pain relievers and fever reducers to help consumers use products safely. Available from URL: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm149573.htm. Accessed 9 Jan 2013.
Cantu C, Arauz A, Murillo-Bonilla LM, et al. Stroke associated with sympathomimetics contained in over-the-counter cough and cold drugs. Stroke. 2003;34(7):1667–72.
McGettigan P, Henry D. Cardiovascular risk with non-steroidal anti-inflammatory drugs: systematic review of population-based controlled observational studies. PLoS Med. 2011;8(9):e1001098.
DuMouchel W. Bayesian data mining in large frequency tables, with an application to the FDA Spontaneous Reporting System. Am Stat. 1999;53:177–202.
Almenoff JS, DuMouchel W, Kindman LA, et al. Disproportionality analysis using empirical Bayes data mining: a tool for the evaluation of drug interactions in the post-marketing setting. Pharmacoepidemiol Drug Saf. 2003;12(6):517–21.
Hauben M, Zhou X. Quantitative methods in pharmacovigilance: focus on signal detection. Drug Saf. 2003;26(3):159–86.
Bousquet C, Henegar C, Louet AL, et al. Implementation of automated signal generation in pharmacovigilance using a knowledge-based approach. Int J Med Inform. 2005;74(7–8):563–71.
Bate A, Edwards IR. Data mining techniques in pharmacovigilance. In: Hartzema AG, Tilson HH, Chan KA, editors. Pharmacoepidemiology and therapeutic risk management. Cincinnati: Harvey Whitney; 2008.
Coulter D. Signal generation in the New Zealand Intensive Medicines Monitoring Programme: a combined clinical and statistical approach. Drug Saf. 2002;25(6):433–9.
Heeley E, Wilton LV, Shakir SA. Automated signal generation in prescription-event monitoring. Drug Saf. 2002;25(6):423–32.
Platt R, Wilson M, Chan KA, et al. The new Sentinel Network: improving the evidence of medical-product safety. N Engl J Med. 2009;361(7):645–7.
Coloma PM, Schuemie MJ, Trifiro G, et al. Combining electronic healthcare databases in Europe to allow for large-scale drug safety monitoring: the EU-ADR Project. Pharmacoepidemiol Drug Saf. 2011;20(1):1–11.
World Health Organization. Safety of medicines: a guide to detecting and reporting adverse drug reactions 2002. Available from URL: http://whqlibdoc.who.int/hq/2002/WHO_EDM_QSM_2002.2.pdf Accessed 10 Jul 2011.
Report of CIOMS Working Group VIII. Practical aspects of signal detection in pharmacovigilance. Geneva: WHO; 2010.
Hauben M, Aronson JK. Defining ‘signal’ and its subtypes in pharmacovigilance based on a systematic review of previous definitions. Drug Saf. 2009;32(2):99–110.
US FDA. FDA Adverse Event Reporting System (AERS). Available from URL: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/default.htm. Accessed 2013 Jan 9.
Vaccine Adverse Event Reporting System. Available from URL: http://vaers.hhs.gov/index/about/index. Accessed 2013 Jan 9.
European Medicines Agency. EudraVigilance. Available from URL: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/document_listing/document_listing_000239.jsp&mid=WC0b01ac05800250b5. Accessed 9 Jan 2013.
European Medicines Agency. 2009 EudraVigilance-human status report. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2010/10/WC500097692.pdf. Accessed 9 Jan 2013.
The Uppsala Monitoring Centre. The WHO programme. Available from URL: http://www.who-umc.org/DynPage.aspx?id=98078&mn1=7347&mn2=7252&mn3=7322. Accessed 20 Apr 2012.
Uppsala Monitoring Centre. Uppsala reports, 2012 April. Available from URL: http://www.who-umc.org/graphics/26656.pdf. Accessed 29 May 2012.
Piccinni C, Sacripanti C, Poluzzi E, et al. Stronger association of drug-induced progressive multifocal leukoencephalopathy (PML) with biological immunomodulating agents. Eur J Clin Pharmacol. 2010;66(2):199–206.
Koutkias V, Niès J, Jensen S, et al., editors. Patient safety informatics: adverse drug events, human factors, and IT tools for patient medication safety, vol. 166. Studies in health technology and informatics. IOS Press; 2011.
Szarfman A, Tonning JM, Doraiswamy PM. Pharmacovigilance in the 21st century: new systematic tools for an old problem. Pharmacotherapy. 2004;24(9):1099–104.
Hauben M, Madigan D, Gerrits CM, et al. The role of data mining in pharmacovigilance. Expert Opin Drug Saf. 2005;4(5):929–48.
Evans SJ, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoeidemiol Drug Saf. 2001;10(6):483–6.
Rothman KJ, Lanes S, Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 2004;13(8):519–23.
Szarfman A, Machado SG, O’Neill RT. Use of screening algorithms and computer systems to efficiently signal higher-than-expected combinations of drugs and events in the US FDA’s spontaneous reports database. Drug Saf. 2002;25(6):381–92.
Bate A, Lindquist M, Edwards IR, et al. A data mining approach for signal detection and analysis. Drug Saf. 2002;25(6):393–7.
Hauben M, Horn S, Reich L. Potential use of data-mining algorithms for the detection of ‘surprise’ adverse drug reactions. Drug Saf. 2007;30(2):143–55.
Vilar S, Harpaz R, Chase HS, et al. Facilitating adverse drug event detection in pharmacovigilance databases using molecular structure similarity: application to rhabdomyolysis. J Am Med Inform Assoc. 2011;18(Suppl. 1):i73–80.
Darpö B. Detection and reporting of drug-induced proarrhythmias: room for improvement. Europace. 2007;9 Suppl. 4:iv23–36.
Blum MD, Graham DJ, McCloskey CA. Temafloxacin syndrome: review of 95 cases. Clin Infect Dis. 1994;18(6):946–50.
Wysowski DK, Swartz L. Adverse drug event surveillance and drug withdrawals in the United States, 1969–2002: the importance of reporting suspected reactions. Arch Intern Med. 2005;165(12):1363–9.
US Food and Drug Administration. FDA announces withdrawal fenfluramine and dexfenfluramine. Available from URL: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm179871.htm. Accessed 18 Oct 2011.
Desmond P. Flucloxacillin hepatitis: an Australian epidemic. Aust NZ J Med. 1995;25(3):195–6.
Salvo F, Polimeni G, Moretti U, et al. Adverse drug reactions related to amoxicillin alone and in association with clavulanic acid: data from spontaneous reporting in Italy. J Antimicrob Chemother. 2007;60(1):121–6.
Thomson JA, Fairley CK, Ugoni AM, et al. Risk factors for the development of amoxycillin-clavulanic acid associated jaundice. Med J Aust. 1995;162(12):638–40.
Withdrawal of rotavirus vaccine recommendation. MMWR Morb Mortal Wkly Rep. 1999;48(43):1007.
Intussusception among recipients of rotavirus vaccine: United States, 1998–1999. MMWR Morb Mortal Wkly Rep. 1999;48(27):577–81.
Murphy TV, Gargiullo PM, Massoudi MS, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med. 2001;344(8):564–72.
Niu MT, Erwin DE, Braun MM. Data mining in the US Vaccine Adverse Event Reporting System (VAERS): early detection of intussusception and other events after rotavirus vaccination. Vaccine. 2001;19(32):4627–34.
Update: Guillain–Barre syndrome among recipients of Menactra meningococcal conjugate vaccine: United States, June 2005–September 2006. MMWR Morb Mortal Wkly Rep. 2006;55(41):1120–4.
Hazell L, Shakir SA. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29(5):385–96.
Friedman MA, Woodcock J, Lumpkin MM, et al. The safety of newly approved medicines: do recent market removals mean there is a problem? JAMA. 1999;281(18):1728–34.
Merck pulls arthritis drug Vioxx from market. Available from URL: http://www.npr.org/templates/story/story.php?storyId=4054991. Accessed 11 Nov 2011.
Krumholz HM, Ross JS, Presler AH, et al. What have we learnt from Vioxx? BMJ. 2007;334(7585):120–3.
Goldman S. Limitations and strengths of spontaneous reports data. Clin Ther. 1998;20 Suppl. C:C40–4.
Trontell A. How the US Food and Drug Administration defines and detects adverse drug events. Curr Ther Res. 2001;62:641–9.
Wang HW, Hochberg AM, Pearson RK, et al. An experimental investigation of masking in the US FDA adverse event reporting system database. Drug Saf. 2010;33(12):1117–33.
Institute of Medicine. The future of drug safety: promoting and protecting the health of the public. Available from URL: http://www.iom.edu/Reports/2006/The-Future-of-Drug-Safety-Promoting-and-Protecting-the-Health-of-the-Public.aspx. Accessed 20 Oct 2011.
Psaty BM, Burke SP. Protecting the health of the public: Institute of Medicine recommendations on drug safety. N Engl J Med. 2006;355(17):1753–5.
Hennessy S. Use of health care databases in pharmacoepidemiology. Basic Clin Pharmacol Toxicol. 2006;98(3):311–3.
Garcia Rodriguez LA, Perez Gutthann S. Use of the UK General Practice Research Database for pharmacoepidemiology. Br J Clin Pharmacol. 1998;45(5):419–25.
Suissa S, Garbe E. Primer: administrative health databases in observational studies of drug effects—advantages and disadvantages. Nat Clin Pract Rheumatol. 2007;3(12):725–32.
Kramarz P, France EK, Destefano F, et al. Population-based study of rotavirus vaccination and intussusception. Pediatr Infect Dis J. 2001;20(4):410–6.
US FDA. The FDA Sentinel initiative. Available from URL: http://www.fda.gov/Safety/FDAsSentinelInitiative. Accessed 12 Jul 2011.
Mini-Sentinel. Available from URL: http://mini-sentinel.org/ Accessed 15 Feb 2011.
Mini-Sentinel. Statistical methods development. Available from URL: http://mini-sentinel.org/methods/methods_development/default.aspx. Accessed 31 May 2012.
Stang PE, Ryan PB, Racoosin JA, et al. Advancing the science for active surveillance: rationale and design for the Observational Medical Outcomes Partnership. Ann Intern Med. 2010;153(9):600–6.
Observational Medical Outcomes Partnership. Health outcomes of interest library. Available from URL: http://omop.fnih.org/HOI. Accessed 11 Nov 2011.
Observational Medical Outcomes Partnership. OMOP Cup 2010. Available from URL: http://omop.fnih.org/omopcup. Accessed 10 Oct 2011.
Observational Medical Outcomes Partnership. OMOP 2011 symposium presentations. Available from URL: http://omop.fnih.org/OMOP2011Symposium. Accessed 30 Mar 2012.
Exploring and Understanding Adverse Drug Reactions by Integrative Mining of Clinical records and Biomedical Knowledge. The EU-ADR Project. Available from URL: http://www.euadr-project.org. Accessed 12 Jul 2011.
Trifiro G, Pariente A, Coloma PM, et al. Data mining on electronic health record databases for signal detection in pharmacovigilance: which events to monitor? Pharmacoepidemiol Drug Saf. 2009;18(12):1176–84.
Platt R, Carnahan RM, Brown JS, et al. The US Food and Drug Administration’s Mini-Sentinel program: status and direction. Pharmacoepidemiol Drug Saf. 2012;21(Suppl. 1):1–8.
Canadian Institutes of Health Research. About the drug safety effectiveness network. Available from URL: http://www.cihr-irsc.gc.ca/e/40269.html. Accessed Mar 2012.
Innovative Medicines Initiative. PROTECT project. Available from URL: http://www.imi-protect.eu/. Accessed Mar 2012.
Global Research in Paediatrics. Available from URL: http://www.grip-network.org/. Accessed 10 May 2012.
Kimura T, Matsushita Y, Yang YH, et al. Pharmacovigilance systems and databases in Korea, Japan, and Taiwan. Pharmacoepidemiol Drug Saf. 2011;20(12):1237–45.
Choi NK, Chang Y, Choi YK, et al. Signal detection of rosuvastatin compared to other statins: data-mining study using national health insurance claims database. Pharmacoepidemiol Drug Saf. 2010;19(3):238–46.
Braitstein P, Einterz RM, Sidle JE, et al. “Talkin’ about a revolution”: how electronic health records can facilitate the scale-up of HIV care and treatment and catalyze primary care in resource-constrained settings. J Acquir Immune Defic Syndr. 2009;52(Suppl. 1):S54–7.
Tierney WM, Achieng M, Baker E, et al. Experience implementing electronic health records in three East African countries. Stud Health Technol Inform. 2010;160(Pt 1):371–5.
Luhm KR, Cardoso MR, Waldman EA. Vaccination coverage among children under two years of age based on electronic immunization registry in Southern Brazil. Rev Saude Publica. 2011;45(1):90–8.
Bate A, Edwards IR, Edwards J, et al. Knowledge finding in IMS disease analyzer Mediplus UK database: effective data mining in longitudinal patient safety data. ISOP Annual Meeting: Pharmacovigilance—Current and Future Challenges, Dublin; 6–8 Oct 2004.
Curtis J, Cheng H, Delzell E, et al. Adaptation of Bayesian data mining algorithms to longitudinal claims data: coxib safety as an example. Med Care. 2008;46(9):969–75.
Wang X, Hripcsak G, Markatou M, et al. Active computerized pharmacovigilance using natural language processing, statistics, and electronic health records: a feasibility study. J Am Med Inform Assoc. 2009;16(3):328–37.
Svanstrom H, Callreus T, Hviid A. Temporal data mining for adverse events following immunization in nationwide Danish healthcare databases. Drug Saf. 2010;33(11):1015–25.
Velentgas P, Bohn RL, Brown JS, et al. A distributed research network model for post-marketing safety studies: the Meningococcal Vaccine Study. Pharmacoepidemiol Drug Saf. 2008;17(12):1226–34.
Lieu TA, Kulldorff M, Davis RL, et al. Real-time vaccine safety surveillance for the early detection of adverse events. Med Care. 2007;45(10 Suppl. 2):S89–95.
Schuemie MJ. Methods for drug safety signal detection in longitudinal observational databases: LGPS and LEOPARD. Pharmacoepidemiol Drug Saf. 2011;20(3):292–9.
Noren GN, Hopstadius J, Bate A, et al. Temporal pattern discovery in longitudinal electronic patient records. Data Min Knowl Disc. 2010;20:361–87.
Observational Medical Outcomes Partnership. OMOP methods library. Available from URL: http://omop.fnih.org/Methods. Accessed 20 May 2012.
Zorych I, Madigan D, Ryan P, et al. Disproportionality methods for pharmacovigilance in longitudinal observational databases. Stat Methods Med Res. 2011 [Epub ahead of print].
Coloma P, Schuemie MJ, Trifiro G, et al. Comparison of methods for drug safety signal detection using electronic healthcare record (EHR) databases: the added value of longitudinal, time-stamped patient information. Presented at the 27th international conference on pharmacoepidemiology and therapeutic risk management, Chicago; 14–17 Aug 2011.
Schuemie MJ, Coloma PM, Straatman H, et al. Using electronic healthcare records for drug safety signal detection: a comparative evaluation of statistical methods. Med Care. 2012;50:890–7.
Bauer-Mehren A, van Mullingen EM, Avillach P, et al. Automatic filtering and substantiation of drug safety signals. PLoS Comput Biol. 2012;8(4):e1002457.
Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58(4):323–37.
Hsia DC, Krushat WM, Fagan AB, et al. Accuracy of diagnostic coding for Medicare patients under the prospective-payment system. N Engl J Med. 1988;318(6):352–5.
Coloma PM, Trifiro G, Schuemie MJ, et al. Electronic healthcare databases for active drug safety surveillance: is there enough leverage? Pharmacoepidemiol Drug Saf. 2012;21:611–21.
Daily Med. Trovafloxacin drug label. Available from URL: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=442&CFID=66575927&CFTOKEN=a17d753a0754a3fb-24987D34-D80B-CD9C-39F668DB8C41A045&jsessionid=ca30e46b22b0112063a4. Accessed 13 Jul 2011.
Daily Med. Rosiglitazone drug label. Available from URL: http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=38243. Accessed 13 Jul 2011.
European Medicines Agency. Tysabri (natalizumab). Available from URL: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000603/human_med_001119.jsp&murl=menus/medicines/medicines.jsp&mid=WC0b01ac058001d124. Accessed 13 Jul 2011.
MHRA. Dopamine agonists for Parkinson’s disease. Available from URL: http://www.mhra.gov.uk/Safetyinformation/Generalsafetyinformationandadvice/Product-specificinformationandadvice/Product-specificinformationandadvice-A-F/DopamineagonistsforParkinson146sdisease/index.htm. Accessed 13 Jul 2011.
European Commission. A guideline on summary of product characteristics. Available from URL: http://ec.europa.eu/health/files/eudralex/vol-2/c/smpc_guideline_rev2_en.pdf. Accessed 20 Apr 2012.
Aronson JK, Ferner RE. Joining the DoTS: new approach to classifying adverse drug reactions. BMJ. 2003;327(7425):1222–5.
Rawlins MD, Thompson JW. Pathogenesis of adverse drug reactions. In: Davies D, editor. Textbook of adverse drug reactions. 3rd ed. Oxford: Oxford University Press; 1985.
Edwards IR. What are the real lessons from Vioxx? Drug Saf. 2005;28(8):651–8.
Fung M, Thornton A, Mybeck K, et al. Evaluation of the characteristics of safety withdrawal of prescription drugs from worldwide pharmaceutical markets—1960 to 1999. Drug Inf J. 2001;35:293–317.
Trifiro G, Patadia V, Schuemie MJ, et al. EU-ADR healthcare database network vs. spontaneous reporting system database: preliminary comparison of signal detection. Stud Health Technol Inform. 2011;166:25–30.
Tatonetti NP, Fernald GH, Altman RB. A novel signal detection algorithm for identifying hidden drug-drug interactions in adverse event reports. J Am Med Inform Assoc. 2012;19(1):79–85.
Meyboom RH, Lindquist M, Egberts AC, et al. Signal selection and follow-up in pharmacovigilance. Drug Saf. 2002;25(6):459–65.
Acknowledgements
The authors would like to thank Dr. Hector Izurieta of the US FDA, and Dr. Jim Slattery of the European Medicines Agency for reviewing the manuscript.
Funding
No sources of funding were used to prepare this manuscript.
Conflict of interest
Miriam Sturkenboom is running a research group that occasionally performs studies for pharmaceutical companies according to unconditional grants. These companies include AstraZeneca, Pfizer, Lilly and Boehringer. She has also been a consultant to Pfizer, Novartis, Consumer Health, Servier, Celgene and Lundbeck on issues not related to this paper. Vaishali Patadia is an employee of Astellas Pharma; the views expressed in this paper are her personal views and do not reflect the views of Astellas Pharma. Preciosa M. Coloma and Gianluca Trifirò have no conflicts of interest to declare that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Coloma, P.M., Trifirò, G., Patadia, V. et al. Postmarketing Safety Surveillance. Drug Saf 36, 183–197 (2013). https://doi.org/10.1007/s40264-013-0018-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-013-0018-x