Abstract
Background and Objective
Rivaroxaban is a novel oral anticoagulant widely used for thromboprophylaxis in patients with non-valvular atrial fibrillation (NVAF). The present study aimed to develop a population pharmacokinetic (PPK) model for rivaroxaban in Chinese patients with NVAF.
Methods
We performed a prospective multicenter study. The plasma concentration of rivaroxaban was directly detected by high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) and indirectly by rivaroxaban-calibrated chromogenic anti-Xa assay (STA®). Gene polymorphisms were detected by MassARRAY single nucleotide polymorphism genotyping technology. Nonlinear mixed-effects modeling was used to develop the PPK model for rivaroxaban in patients with NVAF, and we simulated the steady-state rivaroxaban exposures under different dosing strategies in different covariate levels.
Results
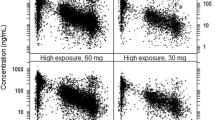
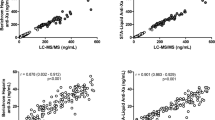
A total of 150 patients from five centers were recruited, including 263 plasma concentrations detected by HPLC-MS/MS, 2626 gene polymorphisms, and 131 plasma concentrations detected by anti-Xa assay. In our study, an oral one-compartment model was used to describe the pharmacokinetics of rivaroxaban in patients with NVAF. In the final model, the estimated apparent clearance (CL/F) and volume of distribution (V/F) were 5.79 L/h (relative standard error [RSE] 4.4%) and 51.5 L (RSE 5.0%), respectively. Covariates in the final model included creatinine clearance, total bilirubin, rs4728709, and body weight. The simulation results showed that in the 15 mg once-daily dosing regimen, in most instances the maximum plasma concentration at steady state (Cmax,ss) and trough plasma concentration at steady state (Cmin,ss) were in the target range for different covariate levels. When patients were administered rivaroxaban 15 or 20 mg once daily, the Cmax,ss and Cmin,ss in the different bodyweight levels were also in the target range. For patients with the ABCB1 rs4728709 mutation, the Cmin,ss in the 10, 15, and 20 mg once-daily dosing regimens were lower than the target range. The anti-Xa assay was highly linearly correlated with the HPLC-MS/MS method [y = 1.014x − 2.4648 (R2 = 0.97)].
Conclusions
Our study was the first multicenter PPK model for rivaroxaban in Chinese patients with NVAF (Alfalfa-RIVAAF-PPK). The study found that 15 mg once daily may be suitable as the principal rivaroxaban dose for Chinese patients with NVAF. For patients with the rs4728709 mutation, it may be necessary to examine insufficient anticoagulation. We found that the rivaroxaban-calibrated chromogenic anti-Xa assay and HPLC-MS/MS method were highly linearly correlated. Prospective studies with larger sample sizes and real-world studies are needed for further verification.



Similar content being viewed by others
References
Mueck W, Stampfuss J, Kubitza D, Becka M. Clinical pharmacokinetic and pharmacodynamic profile of rivaroxaban. Clin Pharmacokinet. 2014;53:1–16.
Kubitza D, Becka M, Voith B, Zuehlsdorf M, Wensing G. Safety, pharmacodynamics, and pharmacokinetics of single doses of BAY 59-7939, an oral, direct factor Xa inhibitor. Clin Pharmacol Ther. 2005;78:412–21.
Gnoth MJ, Buetehorn U, Muenster U, et al. In vitro and in vivo P-glycoprotein transport characteristics of rivaroxaban. J Pharmacol Exp Ther. 2011;338:372–80.
Mueck W, Kubitza D, Becka M. Co-administration of rivaroxaban with drugs that share its elimination pathways: pharmacokinetic effects in healthy subjects. Br J Clin Pharmacol. 2013;76:455–66.
Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KG, et al. 2021 European Heart Rhythm Association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace. 2021;25:euab065.
Blin P, Fauchier L, Dureau-Pournin C, Sacher F, Dallongeville J, Bernard MA, et al. Effectiveness and safety of rivaroxaban 15 or 20 mg versus vitamin K antagonists in nonvalvular atrial fibrillation. Stroke. 2019;50(9):2469–76.
Chen J, Zhang A. Research status and development of atrial fibrillation. J Anhui Health Vocat Tech Coll. 2017;5(16):42–5.
Bounameaux H, Reber G. New oral antithrombotics: a need for laboratory monitoring. Against J Thromb Haemost. 2010;8(4):621–6.
Alalwan AA, Voils SA, Hartzema AG. Trends in utilization of warfarin and direct oral anticoagulants in older adult patients with atrial fibrillation. Am J Health Syst Pharm. 2017;74(16):1237–44.
Testa S, Tripodi A, Legnani C, Pengo V, Abbate R, Dellanoce C, et al. Plasma levels of direct oral anticoagulants in real life patients with atrial fibrillation: Results observed in four anticoagulation clinics. Thromb Res. 2016;137:178–83.
Kanuri SH, Kreutz RP. Pharmacogenomics of novel direct oral anticoagulants: newly identified genes and genetic variants. J Pers Med. 2019;9(1):7.
Eikelboom JW, Quinlan DJ, Hirsh J, Connolly SJ, Weitz JI. Laboratory monitoring of non-vitamin K antagonist oral anticoagulant use in patients with atrial fibrillation: a review. JAMA Cardiol. 2017;2(5):566–74.
Xu XS, Moore K, Burton P, Stuyckens K, Mueck W, Rossenu S, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban in patients with acute coronary syndromes. Br J Clin Pharmacol. 2012;74(1):86–97.
Mueck W, Eriksson BI, Bauer KA, Borris L, Dahl OE, Fisher WD, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban—an oral, direct factor Xa inhibitor—in patients undergoing major orthopaedic surgery. Clin Pharmacokinet. 2008;47(3):203–16.
Mueck W, Borris LC, Dahl OE, Haas S, Huisman MV, Kakkar AK, et al. Population pharmacokinetics and pharmacodynamics of once- and twice-daily rivaroxaban for the prevention of venous thromboembolism in patients undergoing total hip replacement. Thromb Haemost. 2008;100(3):453–61.
Mueck W, Lensing AW, Agnelli G, Decousus H, Prandoni P, Misselwitz F. Rivaroxaban: population pharmacokinetic analyses in patients treated for acute deep-vein thrombosis and exposure simulations in patients with atrial fibrillation treated for stroke prevention. Clin Pharmacokinet. 2011;50(10):675–86.
Willmann S, Zhang L, Frede M, Kubitza D, Mueck W, Schmidt S, et al. Integrated population pharmacokinetic analysis of rivaroxaban across multiple patient populations. CPT Pharmacometrics Syst Pharmacol. 2018;7(5):309–20.
Girgis IG, Patel MR, Peters GR, Moore KT, Mahaffey KW, Nessel CC, et al. Population pharmacokinetics and pharmacodynamics of rivaroxaban in patients with non-valvular atrial fibrillation: results from ROCKET AF. J Clin Pharmacol. 2014;54(8):917–27.
Suzuki S, Yamashita T, Kasai H, Otsuka T, Sagara K. An analysis on distribution and inter-relationships of biomarkers under rivaroxaban in Japanese patients with non-valvular atrial fibrillation (CVI ARO 1). Drug Metab Pharmacokinet. 2018;33(4):188–93.
Feng B. Population pharmacokinetics and pharmacodynamics of rivaroxaban in high-risk patients with thrombosis in my country. Beijing: Peking Union Medical College; 2015.
Chen M, Chen H, Ling WW, Gao HJ, Ling W, Sun H. Population pharmacokinetics of rivaroxaban in patients with nonvalvular atrial fibrillation. J Clin Cardiol. 2020;36(8):724–30.
Zdovc J, Petre M, Pišlar M, Repnik K, Mrhar A, Vogrin M, et al. Downregulation of ABCB1 gene in patients with total hip or knee arthroplasty influences pharmacokinetics of rivaroxaban: a population pharmacokinetic-pharmacodynamic study. Eur J Clin Pharmacol. 2019;75(6):817–24.
Wu TT, Xia XT, Fu JL, Chen WJ, Xiong X, Zhang JH. Simultaneous concentration determination of dabigatran and rivaroxaban in human plasma by LC–MS/MS. Chin J Clin Pharmacol. 2019;35(10):120–3.
Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138(5):1093–100.
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41.
Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12.
Wählby U, Jonsson EN, Karlsson MO. Assessment of actual significance levels for covariate effects in NONMEM. J Pharmacokinet Pharmacodyn. 2001;28:231–52.
Douxfils J, Tamigniau A, Chatelain B, Chatelain C, Wallemacq P, Dogné JM, et al. Comparison of calibrated chromogenic anti-Xa assay and PT tests with LC–MS/MS for the therapeutic monitoring of patients treated with rivaroxaban. Thromb Haemost. 2013;110(4):723–31.
Yang JJ, Cheng C, Devidas M, Cao X, Campana D, Yang W, et al. Genome-wide association study identifies germline polymorphisms associated with relapse of childhood acute lymphoblastic leukemia. Blood. 2012;120(20):4197–204.
Kubitza D, Becka M, Mueck W, et al. Effects of renal impairment on the pharmacokinetics, pharmacodynamics and safety of rivaroxaban, an oral, direct Factor Xa inhibitor. Br J Clin Pharmacol. 2010;70(5):703–12.
Kubitza D, Roth A, Becka M, et al. Effect of hepatic impairment on the pharmacokinetics and pharmacodynamics of a single dose of rivaroxaban, an oral, direct Factor Xa inhibitor. Br J Clin Pharmacol. 2013;76(1):89–98.
Speed V, Green B, Roberts LN, Woolcombe S, Bartoli-Abdou J, Barsam S, et al. Fixed dose rivaroxaban can be used in extremes of bodyweight: a population pharmacokinetic analysis. J Thromb Haemost. 2020;18(9):2296–307.
Ashton V, Mudarris L, Moore KT. The pharmacology, efficacy, and safety of rivaroxaban in obese patient populations. Am J Cardiovasc Drugs. 2021;21(3):283–97.
Kaneko M, Tanigawa T, Hashizume K, Kajikawa M, Tajiri M, Mueck W. Confirmation of model-based dose selection for a Japanese phase III study of rivaroxaban in non-valvular atrial fibrillation patients. Drug Metab Pharmacokinet. 2013;28(4):321–31.
Qian J, Yan YD, Yang SY, Zhang C, Li WY, Gu ZC. Benefits and harms of low-dose rivaroxaban in Asian patients with atrial fibrillation: a systematic review and meta-analysis of real-world studies. Front Pharmacol. 2021;12:642907.
Lin YC, Chien SC, Hsieh YC, Shih CM, Lin FY, Tsao NW, et al. Effectiveness and safety of standard- and low-dose rivaroxaban in Asians with atrial fibrillation. J Am Coll Cardiol. 2018;72(5):477–85.
Samuelson BT, Cuker A, Siegal DM, Crowther M, Garcia DA. Laboratory assessment of the anticoagulant activity of direct oral anticoagulants: a systematic review. Chest. 2017;151(1):127–38.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was supported by grants from the Fujian Provincial Health Technology Project, China (2019-CX-19).
Conflict of interest
Feilong Zhang, Xuehai Chen, Tingting Wu, Nianxu Huang, Li Li, Dongdong Yuan, Jing Xiang, Na Wang, Wenjun Chen, and Jinhua Zhang declare they have no conflicts of interest.
Availability of data and material
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Ethics approval
This study was approved by the hospital Ethics Committee. The procedures used in the present study adhere to the tenets of the Declaration of Helsinki.
Author contributions
FZ, XC, TW: Conceptualization, methodology, investigation, resources, software, validation, writing—original draft. NH, LL, DY, JX, NW, and WC: Investigation, resources, writing—review and editing. JZ: Supervision, project administration, funding acquisition, writing—review and editing.
Consent to participate
Informed consent was obtained from all individual participants included in this study.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, F., Chen, X., Wu, T. et al. Population Pharmacokinetics of Rivaroxaban in Chinese Patients with Non-Valvular Atrial Fibrillation: A Prospective Multicenter Study. Clin Pharmacokinet 61, 881–893 (2022). https://doi.org/10.1007/s40262-022-01108-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-022-01108-3