Background and Objective
Abstract
Therapeutic drug monitoring of tacrolimus whole-blood concentrations is standard care in thoracic organ transplantation. Nevertheless, toxicity may appear with alleged therapeutic concentrations possibly related to variability in unbound concentrations. However, pharmacokinetic data on unbound concentrations are not available. The objective of this study was to quantify the pharmacokinetics of whole-blood, total, and unbound plasma tacrolimus in patients early after heart and lung transplantation.
Methods
Twelve-hour tacrolimus whole-blood, total, and unbound plasma concentrations of 30 thoracic organ recipients were analyzed with high-performance liquid chromatography–tandem mass spectrometry directly after transplantation. Pharmacokinetic modeling was performed using non-linear mixed-effects modeling.
Results
Plasma concentration was < 1% of the whole-blood concentration. Maximum binding capacity of erythrocytes was directly proportional to hematocrit and estimated at 2700 pg/mL (95% confidence interval 1750–3835) with a dissociation constant of 0.142 pg/mL (95% confidence interval 0.087–0.195). The inter-individual variability in the binding constants was considerable (27% maximum binding capacity, and 29% for the linear binding constant of plasma).
Conclusions
Tacrolimus association with erythrocytes was high and suggested a non-linear distribution at high concentrations. Monitoring hematocrit-corrected whole-blood tacrolimus concentrations might improve clinical outcomes in clinically unstable thoracic organ transplants.
Clinical Trial Registration
NTR 3912/EudraCT 2012-001909-24.
Similar content being viewed by others
Tacrolimus is more than 99% associated with erythrocytes. This may result in diminished whole-blood concentrations when hematocrit decreases. |
The whole-blood to unbound plasma concentration ratios differ with changes in hematocrit and show saturation in the higher range of whole-blood tacrolimus concentrations, which may increase toxicity in these higher concentration ranges. |
Because of the complicated bio-analytical challenges, hematocrit-corrected whole-blood concentrations may be the most feasible and suitable surrogate for the prediction of clinical outcomes. |
1 Introduction
Since 1996, tacrolimus has been used as an immunosuppressant in solid organ transplantation. Exposure and outcome relationships of tacrolimus have been extensively studied resulting in a worldwide consensus on its therapeutic window [1]. Nevertheless, there is room for improvement because patients with alleged therapeutic whole-blood concentrations are still at risk of tacrolimus-related toxicity and rejection [2,3,4,5,6]. Tacrolimus extensively binds to red blood cells and blood proteins. As a consequence, tacrolimus whole-blood distribution is strongly affected by hematocrit and protein concentrations, e.g., albumin, lipoproteins, and α1-acid glycoprotein [7,8,9,10,11]. While whole-blood concentrations are commonly used for therapeutic drug monitoring, the unbound tacrolimus plasma concentrations might be better related to the toxicity and efficacy of tacrolimus [8, 12, 13]. In particular, early after heart and lung transplantation, the concentrations of red blood cells and (lipo)proteins show high intra- and interpatient variation [14]. This may give rise to extreme variability in unbound tacrolimus concentrations in the clinically unstable phase after thoracic organ transplantation. Accordingly, therapeutic drug monitoring of unbound tacrolimus plasma concentrations could improve tacrolimus dosing in unstable thoracic organ recipients.
Studies investigating the unbound tacrolimus plasma concentrations are scarce because the quantification of unbound tacrolimus concentrations is bio-analytically challenging and time consuming [7]. As such, the relationship between whole-blood and unbound concentrations has not systematically been studied and no pharmacokinetic models to predict the unbound concentrations based on whole-blood concentrations are available at present. Furthermore, a therapeutic range of unbound tacrolimus plasma concentrations is currently lacking for routine therapeutic drug monitoring [7,8,9, 15].
This study aimed to quantify the pharmacokinetics of whole-blood, total, and unbound plasma tacrolimus in patients early after heart and lung transplantation. With this model, we studied the effect of erythrocyte binding and evaluated whether monitoring based on unbound or total plasma concentrations is feasible as a predictor of clinical outcomes.
2 Methods
Data were derived from 30 thoracic organ transplantation patients comprising ten heart and twenty lung transplantation patients in the first six days after transplantation. The accredited Review Board for Human Studies of the University Medical Center Utrecht approved the study (NTR 3912/EudraCT 2012-001909-24).
2.1 Patients
All thoracic organ recipients admitted to the intensive care unit of the University Medical Center Utrecht between June 2013 and March 2015 were considered for inclusion. Inclusion criteria were patients aged older than 18 years who were treated with tacrolimus and provided informed consent. No patients were excluded because of the exclusion criteria: dying within 1 day after admission, known allergies for tacrolimus and macrolides, or retrieving total parenteral nutrition. The immunosuppressive regimen contained tacrolimus, Prograft® (Astellas Pharma Europe, Leiden, The Netherlands), a cell-cycle blocker, an interleukin-2 inhibitor, and corticosteroids. Tacrolimus was dosed orally twice daily starting with 0.1 mg/kg for the lung recipients and 2 mg for the heart recipients on the day of transplantation. Dose adjustments were based on whole-blood tacrolimus concentrations at 6 a.m. (12 h after administration). The therapeutic window ranged from 9 to 15 ng/mL for all patients.
2.2 Tacrolimus Analyses
Twelve-hour profiles of unbound and total tacrolimus plasma concentrations together with whole-blood tacrolimus concentrations were analyzed daily from the transplantation date until 6 days after transplantation provided the patients were admitted to the intensive care unit. Blood samples were collected between 6 p.m. and 6 a.m. Blood samples for the measurement of unbound and total tacrolimus plasma concentrations were drawn at 0, 2 (or 3 in the case of cystic fibrosis), 6, and 12 hours after administration of tacrolimus and collected in vacutainer tubes of 10 mL containing ethylenediaminetetraacetic acid. Blood samples were immediately centrifuged at the laboratory of the University Medical Center Utrecht. Then, plasma samples were stored at − 80 °C and were analyzed at the end of the study. Analyses of unbound tacrolimus plasma concentrations were performed as described by Stienstra et al. [16]. The method was validated over a linear range of 1.00–200 pg/mL for unbound tacrolimus concentrations in plasma and 100–3200 pg/mL for total plasma concentrations. The lower limit of quantification was 1.00 pg/mL in ultrafiltrate and 100 pg/mL in plasma. The inaccuracy and imprecision for the determination of unbound tacrolimus concentrations in ultrafiltrate and plasma showed a maximum coefficient of variation of 11.7% and a maximum bias of 3.8%.
Analyses of whole-blood tacrolimus was conducted using high-performance liquid chromatography-tandem mass spectrometry (Thermo Fisher Scientific, Eindhoven, The Netherlands) with a lower limit of quantification of 0.5 ng/mL and an intraday imprecision of < 5%. The high-performance liquid chromatography-tandem mass spectrometry method was adapted from and validated according to the latest European Medicines Agency guidelines [17]. The assay has a linear dynamic range of 1–50 ng/mL. Between-run and between-day imprecision (measured by a coefficient of variation) were within 10% and bias was under 3%. Low, median, and high controls were all within 15%. Furthermore, results over 5 years from an international inter-proficiency testing program for tacrolimus showed that all external quality controls were within 15%. The unbound tacrolimus concentrations were quantified using the Thermo Scientific (Waltham, MA, USA) Quantiva LC-MS/MS system with an Ultimate 3000 UHPLC.
2.3 Covariates
Clinical and laboratory data were collected for the study period for: sex, age, reason for transplantation, type of transplantation, length and bodyweight, the Sequential Organ Failure Assessment score, use of extracorporeal membrane oxygenation, administration of red blood cells, concentrations of hematocrit, albumin, high-density lipoproteins, α1-acid glycoprotein, and pH (see also Table 1 and Table S1 of the Electronic Supplementary Material [ESM]).
2.4 Population-Pharmacokinetic Analysis
NONMEM version 7.3.0 was used for modeling tacrolimus pharmacokinetics. The Piraña software program version 2.9.4 was used as an interface for NONMEM and R for Windows version 3.3.1 was used to analyze the results.
2.5 Mixed-Effects Modeling
An open two-compartment linear model with first-order oral absorption best fitted the data. For some dosing occasions, zero order absorption was used. The structural model included the following parameters: clearance, inter-compartmental clearance, distribution volume V1 and V2, and absorption rate constant. The rate of binding of tacrolimus within the central compartment to red blood cells and proteins was considered to be much higher than distribution to the peripheral compartment and elimination. Therefore, whole-blood, total plasma, and unbound plasma concentrations were assumed to be in equilibrium all the time. To incorporate total and unbound plasma concentrations, models for linear binding kinetics (Eq. 1) and models for saturable binding equilibriums (Eq. 2) were tested:
where WBC is whole-blood concentration, Nplasma is the non-specific binding constant, UPC is the unbound tacrolimus plasma concentration, Bmax corresponds to the maximum binding capacity, and Kd is the equilibrium dissociation constant. When the unbound plasma concentration is equal to Kd, the WBC is half of the Bmax.
Total plasma concentrations were similarly related to the unbound plasma concentrations. As tacrolimus is mainly bound to erythrocytes, hematocrit was introduced into the model by multiplying Nplasma or Bmax with the observed hematocrit with the last observation of hematocrit carried forward.Inter-individual variability and inter-occasion variability were described assuming a log normal distribution with the following equation:
where Pkjm is the estimate for parameter k for the jth individual at occasion m, θk is the population value for the kth pharmacokinetic parameter, ηkj represents the inter-individual variability, which is assumed to have a normal distribution with mean 0 and standard deviation ωk, and κkm represents the inter-occasion variability, which is assumed to have a mean 0 and standard deviation of πk. The residual error was assumed to be proportional to the predicted concentration:\(Cij = Cpredij \left( {1 + eij} \right),\),where Cij is the ith observation for the jth individual, Cpredij is the tacrolimus concentration predicted by the model, and eij is the difference between Cij and Cpredij. All values of eij were assumed to be normally distributed with mean 0 and standard deviation σ. Residual error was separately estimated for whole-blood, total, and unbound plasma concentrations. The correlation between these residual error components was estimated using the L2 data option of NONMEM.
The modeling process was performed using the stochastic approximation expectation maximization estimation method with interaction. The likelihood was subsequently established using the Monte Carlo importance sampling estimation method assisted by mode a posteriori. The parameter precision was estimated using the sampling importance resampling procedure [18]. The values of concentrations below the lower limit of quantification were discarded (3.9%; 46 values out of 1180). Model diagnostics were performed by visual checks of standard diagnostic plots, i.e., ‘goodness-of-fit’ plots (see Fig. 1).
2.6 Pharmacokinetic Simulation
The effect of hematocrit on unbound tacrolimus plasma concentrations was assessed by simulation of unbound plasma concentrations for different hematocrit values at a constant WBC (9 ng/mL) using final typical parameter estimates.
2.7 Statistical Analyses
Variables are presented as median (with the first and third quartiles), range, 95% confidence interval (95% CI), or number (proportion) where appropriate.
3 Results
3.1 Patient Characteristics
Ten heart and twenty lung transplantation patients were enrolled in the study and completed the study protocol. Half of the patients were women (15 out of 30; 50%) and the median age was 43 years (range 34–60 years). All heart transplantation patients were diagnosed with dilated cardiomyopathy of whom five patients had ischemic cardiomyopathy and one patient was diagnosed with giant cell myocarditis. Reasons for lung transplantation were cystic fibrosis, chronic obstructive pulmonary disease, idiopathic pulmonary arterial hypertension, idiopathic pulmonary fibrosis, bronchiectasis, Langerhans cell histiocytosis, and sarcoidosis. In Table 1, patient characteristics are summarized. For additional information on clinical characteristics, see Table S1 in the ESM.
3.2 Descriptive Pharmacokinetics
The total number of whole-blood tacrolimus profiles over 0–12 h was 119 with a median of five profiles per patient (range 1–6 profiles). Ninety total and unbound plasma tacrolimus 0- to 12-h profiles were obtained with a median of three per patient (range 0–6). The whole-blood, total, and unbound plasma pharmacokinetic parameters are shown in Table 2. The majority of tacrolimus was associated with erythrocytes as plasma concentrations were < 1% of the whole-blood concentrations. Tacrolimus unbound fraction was < 0.0001 in this population. In Fig. 2a, the observed relationship between unbound plasma and whole-blood concentrations is shown, suggesting non-linear binding of tacrolimus to erythrocytes. The total tacrolimus plasma concentrations showed a linear relationship with the unbound plasma concentrations (see Fig. 2b).
3.3 Model Development
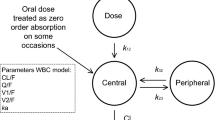
We used a two-compartmental model for whole-blood, total, and unbound plasma tacrolimus concentrations with mixed zero-order and first-order absorption (for a schematic illustration of the pharmacokinetic model, see Fig. 3). To reduce model complexity, the parameters related to absorption and the associated variability were fixed to the previously estimated values. Non-linear binding to erythrocytes and linear binding to plasma proteins best described the data. Furthermore, the Bmax to erythrocytes was directly proportional to hematocrit.
Schematic representation of the population-pharmacokinetic whole-blood concentration (WBC) model for tacrolimus. The central compartment, with volume V1, is swiftly in equilibrium with the peripheral compartment represented by volume V2. Drug transfer between this peripheral compartment and the central compartment is described with the inter-compartmental clearance parameter Q. ka is the absorption rate constant and CL is the whole-blood tacrolimus clearance. The unbound plasma concentration of tacrolimus (UPC) was computed using a non-linear model, as follows: UPC = (WBC × kd1)/(Bmax × Ht − WBC), where kd1 is the dissociation constant (fitted parameter), Bmax is the maximum binding capacity (fitted parameter), and Ht is the observed hematocrit (last observation carried forward). The total plasma concentration (TPC) was computed using a linear model, as follows: TPC Nplasma × UPC with Nplasma non-specific binding constant for total plasma concentrations (fitted parameter)
Table 3 shows the parameter estimates and precision of the final model. The parameter precision was acceptable for all relevant parameters, as represented by the small 95% CI of the parameter estimates. The residual unexplained variability was low for the whole-blood concentrations at 16.7% (95% CI 15.8–17.6) and higher for the unbound concentrations at 36.3% (95% CI 33.9–40.4) and total plasma concentrations at 31.6% (95% CI 28.6–34.2). Substantial correlation of residual variability between whole-blood and plasma concentration was found as expected. Goodness-of-fit plots of unbound tacrolimus plasma concentrations exhibited widely dispersed data around the unity line.
3.4 Results of Pharmacokinetic Modeling and Simulations
Large inter-occasion variability in bioavailability was found, which dominates inter-individual variability (see Table 3). The dissociation constant of distribution to erythrocytes was estimated at 0.142 pg/mL (95% CI 0.087–0.195), which is relatively high and only indicates a slight non-linear distribution relevant at high tacrolimus concentrations. However, this model was superior to a linear binding model. The Nplasma for total plasma concentrations was estimated at 137 (95% CI 120–152), indicating that total plasma concentrations were typically 137-fold higher than unbound plasma concentrations. Moreover, the inter-individual variability in the binding constants was also considerable (27% for Bmax WBC and 29% for Nplasma).
Simulations of different hematocrit ratios ranging from 0.25 to 0.50 at a fixed WBC of 9 ng/mL were conducted. The unbound concentration decreased with increasing hematocrit and ranged from 1.06 to 2.14 pg/mL. When the hematocrit declined from 0.45 to 0.30, the unbound tacrolimus plasma concentration showed to be 1.5 times higher. A non-linear relationship between unbound plasma and hematocrit was observed as shown in Fig. 4.
4 Discussion
This is the first report of a population-pharmacokinetic model of whole-blood, total, and unbound tacrolimus plasma concentrations. Accumulation of tacrolimus in erythrocytes was high relative to plasma concentrations. The whole-blood to unbound plasma concentration ratios differed with changes in hematocrit and showed saturation in the higher range of whole-blood tacrolimus concentrations. Consequently, the combination of high whole-blood tacrolimus concentrations with low hematocrit concentrations may result in extremely high unbound plasma concentrations and hence, in toxicity. From a theoretical perspective, the unbound tacrolimus plasma concentrations would be a better surrogate for the prediction of clinical outcomes. Yet, the analysis of unbound tacrolimus plasma concentrations is challenging and not easily standardized. As a linear relationship between total and unbound tacrolimus plasma concentrations was found, measurement of total plasma concentrations may be considered as an alternative predictive biomarker of clinical outcomes. However, the accuracy and precision of plasma tacrolimus concentration quantification are vulnerable to hemolysis of whole-blood samples. To circumvent these bio-analytical challenges, hematocrit-corrected whole-blood concentrations may be the most feasible and suitable surrogate for the prediction of clinical outcomes.
The whole-blood concentrations far exceeded total plasma concentrations, indicating that tacrolimus mainly distributes within erythrocytes. This is in line with earlier reports, although the plasma-to-blood ratio we found was considerably lower [8, 15, 19]. We observed a large variability in unbound tacrolimus plasma concentrations. Moreover, the observed high inter-individual variability in the binding constants (Bmax WBC and Nplasma) indicates highly variable binding of tacrolimus in the central compartment. It has been shown that tacrolimus whole-blood apparent clearance is inversely correlated to hematocrit and erythrocyte count [9, 20,21,22]. Although comparable with a study in pregnant women, we observed a low median and wide range in hematocrit in this population compared with studies after liver and kidney transplantation [8, 9, 12, 23]. This may be explained by frequent major bleedings peri-operatively, bone marrow depression due to inflammation, blood cell transfusions needed to optimize oxygen demand, and hemolysis due to the use of extracorporeal equipment, which are all common events in thoracic transplant patients in the early post-transplant phase. Moreover, we observed a non-linear relationship between whole-blood and unbound tacrolimus plasma concentrations suggesting saturation of erythrocytes. The relatively high dissociation constant indicates that binding to erythrocytes was only slightly non-linear in the observed unbound concentration range, which is substantiated by the relative large CI of this parameter. Although suggested by in vitro observations, saturation has never been observed in vivo before [8, 24, 25]. A combination of high tacrolimus concentrations with low hematocrit concentrations may result in excessively high unbound concentrations and consequently lead to tacrolimus-related toxicity.
Interestingly, the Bmax of erythrocytes showed wide inter-patient variability. Different protein content within erythrocytes may explain this finding. Tacrolimus is bound to the cytoplasmic FK506 binding protein 12 and to a lesser extent to the membrane-associated FK506 binding protein 13 [19, 26,27,28]. Saturation of these proteins within the erythrocyte is the most logical explanation for this effect [28, 29].
The simulation of unbound tacrolimus concentrations with different hematocrit and fixed WBC indicated that patients with a low hematocrit tend to have lower whole-blood concentrations, whilst the unbound concentration is around the population mean. In practice, transplant physicians will increase the dose, even though the unbound concentrations as well as the total plasma concentrations will increase as shown by our data. Increasing the dose may lead to higher toxicity by higher unbound concentrations. On the contrary, the risk for rejection is low early after lung transplantation while prevention from cellular and antibody-mediated rejection is also controlled with the addition of induction therapy with an interleukin-2 receptor antagonist, anti-thymocyte globulins, or anti-CD52 monoclonal antibodies next to triple therapy with corticosteroids, an anti-proliferative agent, and tacrolimus [30]. However, a low WBC does not imply a low unbound plasma concentration. The simulation showed that a decline in hematocrit, e.g., from 0.45 to 0.30, indicates an increase in the unbound tacrolimus plasma concentrations from 1.15 up to 1.77 pg/mL, which is 1.5 times higher. For clinical practice, this could mean that, at a constant WBC of 9 ng/mL and a decline of hematocrit from 0.45 to 0.30, lowering the dose by 33% should be efficacious to control the unbound tacrolimus plasma concentration.
Importantly, sample procurement may highly influence unbound tacrolimus plasma concentrations. Swift analysis of the samples is important as well as incubation and centrifugation temperatures. Hemolysis may influence the plasma concentrations whilst no difference was found between analyses at 20 °C or 37 °C [15, 31, 32]. In this study, blood samples were immediately centrifuged to minimize erythrocyte damage and tacrolimus distribution from red blood cells to plasma. Importantly, at lower temperatures (4 °C), affinity to erythrocytes is higher than at room and body temperatures [31]. In this study, temperature was kept constant during centrifugation and subsequent filtration at 25 °C to diminish a temperature-dependent effect on the binding of tacrolimus to erythrocytes [16]. This vulnerability to hemolysis makes analyses of plasma tacrolimus concentrations as a routine practice a major challenge. Our results concur with previous studies that suggest hematocrit-corrected whole-blood concentrations could be of use for improved target exposure [11, 24, 33]. We assume that only correction to hematocrit is sufficient while protein levels will not have a large effect on the whole-blood concentrations because the majority of tacrolimus was associated with erythrocytes and plasma concentrations were < 1% of the whole-blood concentrations. The advantage of monitoring hematocrit-corrected whole-blood concentrations over unbound or total plasma concentrations is that it may be easily implemented in daily transplantation practice.
The strong aspects of this study are the use of full 12-h profiles, the use of high-performance liquid chromatography with tandem mass spectrometry for analyses of tacrolimus concentrations, and the use of non-linear mixed-effects modeling for pharmacokinetic modeling. Earlier studies on unbound concentrations were mostly performed with immuno-assays, which in itself have large variations and may have unreliable results especially in the low range of the unbound concentrations [7, 9, 34].
Although a relatively small group of patients was included, the number of tacrolimus profiles was sufficient to perform pharmacokinetic modeling. We did not examine any relationships between the pharmacokinetics and pharmacodynamics of unbound and total tacrolimus plasma concentrations in this study. Whether hematocrit-corrected dosing diminishes the variability in unbound plasma concentrations and subsequent toxicity needs further validation.
5 Conclusions
A two-compartment pharmacokinetic model was designed with mixed zero and first-order absorption of tacrolimus whole-blood, total, and unbound plasma concentrations in thoracic organ recipients in the first week after transplantation. The unbound concentration was mainly influenced by the variability in erythrocyte count. Erythrocyte binding was saturable. Subsequently, the total or unbound tacrolimus plasma concentrations might be better predictors of clinical outcomes. Nevertheless, robust bio-analysis of the unbound tacrolimus plasma as well as the total plasma concentrations is challenging. Therefore, hematocrit-corrected whole-blood concentrations may serve as the most feasible and suitable predictive exposure measure to improve clinical outcomes and should be further explored in future studies.
Change history
01 March 2022
A Correction to this paper has been published: https://doi.org/10.1007/s40262-022-01115-4
References
Wallemacq P, Armstrong VW, Brunet M, Haufroid V, Holt DW, Johnston A, et al. Opportunities to optimize tacrolimus therapy in solid organ transplantation: report of the European consensus conference. Ther Drug Monit. 2009;2009(31):139–52. https://doi.org/10.1097/ftd.0b013e318198d092.
Rayar M, Tron C, Jézéquel C, Beaurepaire J-M, Petitcollin A, Houssel-Debry P, et al. High intrapatient variability of tacrolimus exposure in the early period after liver transplantation is associated with poorer outcomes. Transplantation. 2018;102:e108–14. https://doi.org/10.1097/tp.0000000000002052.
Gueta I, Markovits N, Yarden-Bilavsky H, Raichlin E, Freimark D, Lavee J, et al. High tacrolimus trough level variability is associated with rejections after heart transplant. Am J Transplant. 2018;18:2571–8. https://doi.org/10.1111/ajt.15016.
Bouamar R, Shuker N, Hesselink DA, Weimar W, Ekberg H, Kaplan B, et al. Tacrolimus predose concentrations do not predict the risk of acute rejection after renal transplantation: a pooled analysis from three randomized-controlled clinical trials(†). Am J Transplant. 2013;13:1253–61. https://doi.org/10.1111/ajt.12191.
Sikma MA, Hunault CC, Kirkels JH, Verhaar MC, Kesecioglu J, de Lange DW. Association of whole blood tacrolimus concentrations with kidney injury in heart transplantation patients. Eur J Drug Metab Pharmacokinet. 2018;43:311–20. https://doi.org/10.1007/s13318-017-0453-7.
Sikma MA, Hunault CC, van de Graaf EA, Verhaar MC, Kesecioglu J, de Lange DW, et al. High tacrolimus blood concentrations early after lung transplantation and the risk of kidney injury. Eur J Clin Pharmacol. 2017;73:573–80. https://doi.org/10.1007/s00228-017-2204-8.
Zahir H, Nand RA, Brown KF, Tattam BN. Validation of methods to study the distribution and protein binding of tacrolimus in human blood. J Pharmacol Toxicol Methods. 2001;46:27–35. https://doi.org/10.1016/s1056-8719(02)00158-2.
Zahir H, McCaughan G, Gleeson M, Nand RA, McLachlan AJ. Factors affecting variability in distribution of tacrolimus in liver transplant recipients. Br J Clin Pharmacol. 2004;57:298–309. https://doi.org/10.1046/j.1365-2125.2003.02008.x.
Zheng S, Easterling TR, Umans JG, Miodovnik M, Calamia JC, Thummel KE, Shen DD, Davis CL, Hebert MF. Pharmacokinetics of tacrolimus during pregnancy. Ther Drug Monit. 2012;34:660–70. https://doi.org/10.1097/ftd.0b013e3182708edf
Brooks E, Tett SE, Isbel NM, Staatz CE. Population pharmacokinetic modelling and Bayesian estimation of tacrolimus exposure: is this clinically useful for dosage prediction yet? Clin Pharmacokinet. 2016;55:1295–335. https://doi.org/10.1007/s40262-016-0396-1.
Schijvens AM, van Hesteren FHS, Cornelissen EAM, Bootsma-Robroeks CMHHT, Brüggemann RJM, Burger DM, et al. The potential impact of hematocrit correction on evaluation of tacrolimus target exposure in pediatric kidney transplant patients. Pediatr Nephrol. 2019;34:507–15. https://doi.org/10.1007/s00467-018-4117-x.
Zahir H, McCaughan G, Gleeson M, Nand RA, McLachlan AJ. Changes in tacrolimus distribution in blood and plasma protein binding following liver transplantation. Ther Drug Monit. 2004;26:506–15. https://doi.org/10.1097/00007691-200410000-00008.
Hebert MF, Zheng S, Hays K, Shen DD, Davis CL, Umans JG, et al. Interpreting tacrolimus concentrations during pregnancy and postpartum. Transplantation. 2013;95:908–15. https://doi.org/10.1097/TP.0b013e318278d367.
Sikma MA, van Maarseveen EM, van de Graaf EA, Kirkels JH, Verhaar MC, Donker DW, et al. Pharmacokinetics and toxicity of tacrolimus early after heart and lung transplantation. Am J Transplant. 2015;15:2301–13. https://doi.org/10.1111/ajt.13309.
Bittersohl H, Schniedewind B, Christians U, Luppa PB. A simple and highly sensitive on-line column extraction liquid chromatography–tandem mass spectrometry method for the determination of protein-unbound tacrolimus in human plasma samples. J Chromatogr A. 2018;1547:45–52. https://doi.org/10.1016/j.chroma.2018.03.010.
Stienstra NA, Sikma MA, van Dapperen AL, de Lange DW, Van Maarseveen EM. Development of a simple and rapid method to measure the free fraction of tacrolimus in plasma using ultrafiltration and LC–MS/MS. Ther Drug Monit. 2016;38:722–7. https://doi.org/10.1097/FTD.0000000000000351.
Marinova M, Artusi C, Brugnolo L, Antonelli G, Zaninotto M, Plebani M. Immunosuppressant therapeutic drug monitoring by LC–MS/MS: workflow optimization through automated processing of whole blood samples. Clin Biochem. 2013;46:1723–7. https://doi.org/10.1016/j.clinbiochem.2013.08.013.
Dosne A-G, Bergstrand M, Harling K, Karlsson MO. Improving the estimation of parameter uncertainty distributions in nonlinear mixed effects models using sampling importance resampling. J Pharmacokinet Pharmacodyn. 2016;43:583–96. https://doi.org/10.1007/s10928-016-9487-8.
Nagase K, Iwasaki K, Nozaki K, Noda K. Distribution and protein binding of FK506, a potent immunosuppressive macrolide lactone, in human blood and its uptake by erythrocytes. J Pharm Pharmacol. 1994;46:113–7. https://doi.org/10.1111/j.2042-7158.1994.tb03752.x.
Benkali K, Prémaud A, Picard N, Rérolle J-P, Toupance O, Hoizey G, et al. Tacrolimus population pharmacokinetic–pharmacogenetic analysis and Bayesian estimation in renal transplant recipients. Clin Pharmacokinet. 2009;48:805–16. https://doi.org/10.2165/11318080-000000000-00000.
De Jonge H, Vanhove T, de Loor H, Verbeke K, Kuypers DRJ. Progressive decline in tacrolimus clearance after renal transplantation is partially explained by decreasing CYP3A4 activity and increasing haematocrit. Br J Clin Pharmacol. 2015;80:548–59. https://doi.org/10.1111/bcp.12703.
Andrews LM, Hesselink DA, Van Gelder T, Koch BCP, Cornelissen EAM, Brüggemann RJM, et al. A population pharmacokinetic model to predict the individual starting dose of tacrolimus following pediatric renal transplantation. Clin Pharmacokinet. 2018;57:475–89. https://doi.org/10.1007/s40262-017-0567-8.
Størset E, Holford N, Hennig S, Bergmann TK, Bergan S, Bremer S, et al. Improved prediction of tacrolimus concentrations early after kidney transplantation using theory-based pharmacokinetic modelling. Br J Clin Pharmacol. 2014;78:509–23. https://doi.org/10.1111/bcp.12361.
Størset E, Holford N, Midtvedt K, Bremer S, Bergan S, Åsberg A. Importance of hematocrit for a tacrolimus target concentration strategy. Eur J Clin Pharmacol. 2014;70:65–77. https://doi.org/10.1007/s00228-013-1584-7.
Chow F-S, Piekoszewski W, Jusko WJ. Effect of hematocrit and albumin concentration on hepatic clearance of tacrolimus (FK506) during rabbit liver perfusion. Drug Metab Dispos. 1997;25:610–6.
Biagiotti S, Paoletti MF, Fraternale A, Rossi L, Magnani M. Drug delivery by red blood cells. IUBMB Life. 2011;63:621–31. https://doi.org/10.1002/iub.478.
Biagiotti S, Rossi L, Bianchi M, Giacomini E, Pierigè F, Serafini G, et al. Immunophilin-loaded erythrocytes as a new delivery strategy for immunosuppressive drugs. J Control Release. 2011;154:306–13. https://doi.org/10.1016/j.jconrel.2011.05.024.
Walensky LD, Gascard P, Fields ME, Blackshaw S, Conboy JG, Mohandas N, et al. The 13-kD FK506 binding protein, FKBP13, interacts with a novel homologue of the erythrocyte membrane cytoskeletal protein 4.1. J Cell Biol. 1998;141:143–53. https://doi.org/10.1083/jcb.141.1.143.
Van Acker K, Bultynck G, Rossi D, Sorrentino V, Boens N, Missiaen L, et al. The 12 kDa FK506-binding protein, FKBP12, modulates the Ca(2 +)-flux properties of the type-3 ryanodine receptor. J Cell Sci. 2004;117:1129–37. https://doi.org/10.1242/jcs.00948.
Scheffert JL, Raza K. Immunosuppression in lung transplantation. J Thorac Dis. 2014;6:1039–53. https://doi.org/10.3978/j.issn.2072-1439.2014.04.23.
Machida M, Takahara S, Ishibashi M, Hayashi M. Effect of temperature on hematocrit on plasma concentration of FK506. Transplant Proc. 1991;23:2753–4. https://doi.org/10.5980/jpnjurol1989.84.1088.
Beysens AJ, Wijnen RM, Beuman GH, van der Heyden J, Kootstra G, van As H. FK 506: monitoring in plasma or in whole blood? Transplant Proc. 1991;23:2745–7.
Gérard C, Stocco J, Hulin A, Blanchet B, Verstuyft C, Durand F, et al. Determination of the most influential sources of variability in tacrolimus trough blood concentrations in adult liver transplant recipients: a bottom-up approach. AAPS J. 2014;16:379–91. https://doi.org/10.1208/s12248-014-9577-8.
Piekoszewski W, Jusko WJ. Plasma protein binding of tacrolimus in humans. J Pharm Sci. 1993;82:340–1. https://doi.org/10.1002/jps.2600820325.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were received for the conduct of this study of the preparation of this article.
Conflict of interest
Jozef Kesecioglu reports personal fees from an honorarium received from Xenios AG, outside the submitted work. Maaike A. Sikma, Erik M. Van Maarseveen, Claudine C. Hunault, Javier M. Moreno, Ed A. Van de Graaf, Johannes H. Kirkels, Marianne C. Verhaar, Jan C. Grutters, Dylan W. De Lange, and Alwin D.R. Huitema have no conflicts of interest that are directly relevant to the content of this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sikma, M.A., Van Maarseveen, E.M., Hunault, C.C. et al. Unbound Plasma, Total Plasma, and Whole-Blood Tacrolimus Pharmacokinetics Early After Thoracic Organ Transplantation. Clin Pharmacokinet 59, 771–780 (2020). https://doi.org/10.1007/s40262-019-00854-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-019-00854-1