Abstract
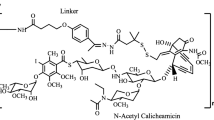
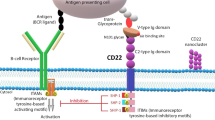
CD22 is a B-cell-specific transmembrane glycoprotein found on the surface of most B cells; it modulates B-cell function, survival and apoptosis. CD22 has emerged as an ideal target for monoclonal antibody (mAb)-based therapy of B-cell malignancies including most lymphomas and many leukemias. Epratuzumab, an anti-CD22 mAb, has been developed in various forms, including as an unlabeled (naked) mAb, as a radioimmunotherapeutic, as an antibody drug conjugate (ADC), and as a vehicle for CD22-targeted nanoparticles. While clinical trials with unlabeled epratuzumab have demonstrated modest results, its combination with rituximab in phase II studies has been more encouraging. Based on the potential for CD22 to become internalized, CD22-targeted constructs carrying radioisotopes or toxins have generated promising results. Radioimmunotherapy, utilizing 90Y-labeled epratuzumab, was shown to be highly effective in patients with follicular lymphoma, generating a complete response (CR) rate of 92 % and progression-free survival of more than 2 years. ADC therapy is a promising therapeutic approach to B-cell malignancies which includes the direct conjugation of mAbs with cytotoxic agents. Phase II studies of inotuzumab ozogamicin, an ADC which combines anti-CD22 mAb with calicheamicin, an enediyne antibiotic which mediates apoptosis, in patients with acute lymphoblastic leukemia have produced an overall response rate (ORR) of greater than 50 % in treatment-refractory patients. Phase I trials of moxetumomab pasudotox, an ADC which combines anti-CD22 with PE38, a fragment of Pseudomonas exotoxin A, have been completed in hairy cell leukemia with a ORR of 86 %. Finally, a review of CD22-targeted nanoparticles, that include a doxorubicin-containing lipid complex that uses synthetic high-affinity CD22 ligand mimetics as well as anti-CD22 mAb-coated pegylated liposomas doxorubin (PLD), has demonstrated promising results in pre-clinical models of human lymphoma. Moreover, novel anti-CD22 mAb that block CD22 ligand binding as well as second generation ADC that utilize biodegradable linkers and more potent toxins hold great hope for the future of CD22-targeted therapeutics that may translate into better outcomes for patients with CD22-positive malignancies.
Similar content being viewed by others
References
Walker JA, Smith KGC. CD22: an inhibitory enigma. Immunology. 2008;123(3):314–25.
Engel P, Wagner N, Miller AS, Tedder TF. Identification of the ligand-binding domains of the CD22, a member of the immunoglobulin superfamily that uniquely binds a sialic acid-dependent ligand. J Exp Med. 1995;181:1581–6.
Otipoby KL, Draves KE, Clark EA. CD22 regulates B cell receptor-mediated signals via two domains that independently recruit Grb2 and SHP-1. J Biol Chem. 2001;276(47):44315–22.
Fujimoto M, Kuwano Y, Watanabe R, et al. B cell antigen receptor and CD40 differentially regulate CD22 tyrosine phosphorylation. J Immunol. 2006;176:873–9.
Gross AJ, Lyandres JR, Panigrahi AK, Prak ET, DeFranco AL. Developmental acquisition of the Lyn-CD22-SHP-1 inhibitory pathway promotes B cell tolerance. J Immunol. 2009;182(9):5382–92.
Xu Y, Harder KW, Huntington ND, Hibbs ML, Tarlinton DM. Lyn tyrosine kinase: accentuating the positive and the negative. Immunity. 2005;22:9–18.
Gerlach J, Ghosh S, Jumaa H, Reth M, Wienands J, Chan AC, Nitschke L. B cell defects in SLP65/BLNK-deficient mice can be partially corrected by the absence of CD22, an inhibitory coreceptor for BCR signaling. Eur J Immunol. 2003;33:3418–26.
Jellusova J, Nitschke L. Regulation of B cell functions by the sialic acid-binding receptors siglec-G and CD22. Front Immunol. 2011;2:96.
Carnahan J, Stein R, Qu Z, Hess K, Cesano A, Hansen HJ, Goldenberg DM. Epratuzumab, a CD22-targeting recombinant humanized antibody with a different mode of action from rituximab. Mol Immunol. 2007;44(6):1331–41. Epub 2006 Jun 30.
Leung SO, Shevitz J, Pellegrini MC, Dion AS, Shih LB, Goldenberg DM, Hansen HJ. Chimerization of LL2, a rapidly internalizing antibody specific for B cell lymphoma. Hybridoma. 1994;13(6):469–76.
Losman MJ, Hansen HJ, Dworak H, Krishnan IS, Qu Z, Shih LB, Zeng L, Goldenberg DM, Leung SO. Generation of a high-producing clone of a humanized anti-B-cell lymphoma monoclonal antibody (hLL2). Cancer. 1997;80(12 Suppl):2660–6.
Leonard JP, Link BK. Immunotherapy of non-Hodgkin’s lymphoma with hLL2 (epratuzumab, an anti-CD22 monoclonal antibody) and Hu1D10 (apolizumab). Semin Oncol. 2002;29(1 Suppl 2):81–6.
Tuscano JM, O’Donnell RT, Miers LA, Kroger LA, Kukis DL, Lamborn KR, Tedder TF, DeNardo GL. Anti-CD22 ligand-blocking antibody HB22.7 has independent lymphomacidal properties and augments the efficacy of 90Y-DOTA-peptide-Lym-1 in lymphoma xenografts. Blood. 2003;101(9):3641–7. Epub 2003 Jan 2.
O’Donnell RT, Ma Y, McKnight HC, Pearson D, Tuscano JM. Dose, timing, schedule, and the choice of targeted epitope alter the efficacy of anti-CD22 immunotherapy in mice bearing human lymphoma xenografts. Cancer Immunol Immunother. 2009;58(12):2051–8. Epub 2009 May 13.
Leonard JP, Coleman M, Ketas JC, Chadburn A, Ely S, Furman RR, Wegener WA, Hansen HJ, Ziccardi H, Eschenberg M, Gayko U, Cesano A, Goldenberg DM. Phase I/II trial of epratuzumab (humanized anti-CD22 antibody) in indolent non-Hodgkin’s lymphoma. J Clin Oncol. 2003;21(16):3051–9. Epub 2003 Jul 1.
Leonard JP, Coleman M, Ketas JC, Chadburn A, Furman R, Schuster MW, Feldman EJ, Ashe M, Schuster SJ, Wegener WA, Hansen HJ, Ziccardi H, Eschenberg M, Gayko U, Fields SZ, Cesano A, Goldenberg DM. Epratuzumab, a humanized anti-CD22 antibody, in aggressive non-Hodgkin’s lymphoma: phase I/II clinical trial results. Clin Cancer Res. 2004;10(16):5327–34.
Rossi EA, Michel R, Wallace DJ, Chang CH, Goldenberg DM. Targeting epratuzumab down-regulates multiple BCR regulators on the surface of normal, lupus and malignant B cells. Presented at the 54th ASH Annual Meeting and Exposition, Atlanta, GA, 8–11 December 2012.
Raetz EA, Cairo MS, Borowitz MJ, Blaney SM, Krailo MD, Leil TA, Reid JM, Goldenberg DM, Wegener WA, Carroll WL, Adamson PC. Chemoimmunotherapy reinduction with epratuzumab in children with acute lymphoblastic leukemia in marrow relapse: a children’s oncology group pilot study. J Clin Oncol. 2008;26(22):3756–62.
Raetz EA, Cairo MS, Borowitz MJ, Lu X, Dvidas M, Reid JM, Goldenberg DM, Wegener WA, Whitlock JA, Adamson PC, Hunger SP, Carroll WL. Reinduction chemoimmunotherapy with epratuzumab in relapsed acute lymphoblastic leukemia (ALL) in children, adolescents and young adults: results from Children’s Oncology Group (COG) Study ADVL04P2. Presented at the 53rd ASH Annual Meeting, 10–13 December 2011, San Diego, CA.
Raetz EA, Borowitz MJ, Devidas M, Linda SB, Hunger SP, Winick NJ, Camitta BM, Gaynon PS, Carroll WL. Reinduction platform for children with first marrow relapse of acute lymphoblastic Leukemia: A Children’s Oncology Group Study [corrected]. J Clin Oncol. 2008;26(24):3971–8. doi:10.1200/JCO.2008.16.1414.
Phase II, multicenter, open label, prospective to evaluate efficacy and tolerance of a chemoimmunotherapy with hyperCVAD or vincristine/dexamethasone plus the anti-CD22 monoclonal antibody epratuzumab for the treatment of adult relapsed/refractory CD22+ B-acute lymphoblastic leukaemia patients: CHEPRALL Study, a GRAALL Study. Estimated primary completion date: January 2013.
Advani A, McDonough S, Coutre S, Wood BL, Radich JP, Mims M, O’Donnell M, Elkins S, Becker MW, Othus M, Appelbaum FR. Southwest Oncology Group Study S0910: a phase 2 trial of clofarabine/cytarabine/epratuzumab for relapsed/refractory acute lymphocytic leukemia. Presented at the 54th ASH Annual Meeting and Exposition, Atlanta, GA, 8–11 December, 2012.
Micallef IN, Maurer MJ, Wiseman GA, Nikcevich DA, Kurtin PJ, Cannon MW, Perez DG, Soori GS, Link BK, Habermann TM, Witzig TE. Epratuzumab with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy in patients with previously untreated diffuse large B-cell lymphoma. Blood. 2011;118(15):4053–61. Epub 2011 Jun 14.
Leonard JP, Coleman M, Ketas J, Ashe M, Fiore JM, Furman RR, Niesvizky R, Shore T, Chadburn A, Horne H, Kovacs J, Ding CL, Wegener WA, Horak ID, Goldenberg DM. Combination antibody therapy with epratuzumab and rituximab in relapsed or refractory non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23(22):5044–51.
Strauss SJ, Morschhauser F, Rech J, Repp R, Solal-Celigny P, Zinzani PL, Engert A, Coiffier B, Hoelzer DF, Wegener WA, Teoh NK, Goldenberg DM, Lister TA. Multicenter phase II trial of immunotherapy with the humanized anti-CD22 antibody, epratuzumab, in combination with rituximab, in refractory or recurrent non-Hodgkin’s lymphoma. J Clin Oncol. 2006;24(24):3880–6.
Leonard JP, Schuster SJ, Emmanouilides C, Couture F, Teoh N, Wegener WA, Coleman M, Goldenberg DM. Durable complete responses from therapy with combined epratuzumab and rituximab: final results from an international multicenter, phase 2 study in recurrent, indolent, non-Hodgkin lymphoma. Cancer. 2008;113(10):2714–23.
Solal-Celigny P, Roy P, Colombat P, et al. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–65.
Micallef IN, Maurer MJ, Wiseman GA, Nikcevich DA, Kurtin PJ, Cannon MW, Perez DG, Soori GS, Link BK, Habermann TM, Witzig TE. Epratuzumab with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy in patients with previously untreated diffuse large B-cell lymphoma. Blood. 2011;118(15):4053–61. Epub 2011 Jun 14.
Ribrag V, Gisselbrecht C, Haioun C, Salles G, Golfier JB, Ertault M, Ferme C, Briere J, Brice P, Mounier N. Efficacy and toxicity of 2 schedules of frontline rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone plus bortezomib in patients with B-cell lymphoma: a randomized phase 2 trial from the French Adult Lymphoma Study Group (GELA). Cancer. 2009;115(19):4540–6.
Nowakowski GS, Maurer MJ, Habermann TM, Ansell SM, Macon WR, Ristow KM, Allmer C, Slager SL, Witzig TE, Cerhan JR. Statin use and prognosis in patients with diffuse large B-cell lymphoma and follicular lymphoma in the rituximab era. J Clin Oncol. 2010;28(3):412–7. Epub 2009 Dec 14.
Drake MT, Maurer MJ, Link BK, Habermann TM, Ansell SM, Micallef IN, Kelly JL, Macon WR, Nowakowski GS, Inwards DJ, Johnston PB, Singh RJ, Allmer C, Slager SL, Weiner GJ, Witzig TE, Cerhan JR. Vitamin D insufficiency and prognosis in non-Hodgkin’s lymphoma. J Clin Oncol. 2010;28(27):4191–8. Epub 2010 Aug 16.
Sharkey RM, Brenner A, Burton J, Hajjar G, Toder SP, Alavi A, Matthies A, Tsai DE, Schuster SJ, Stadtmauer EA, Czuczman MS, Lamonica D, Kraeber-Bodere F, Mahe B, Chatal JF, Rogatko A, Mardirrosian G, Goldenberg DM. Radioimmunotherapy of non-Hodgkin’s lymphoma with 90Y-DOTA humanized anti-CD22 IgG (90Y-Epratuzumab): do tumor targeting and dosimetry predict therapeutic response? J Nucl Med. 2003;44(12):2000–18.
Press OW, Eary JF, Appelbaum FR, Martin PJ, Badger CC, Nelp WB, Glenn S, Butchko G, Fisher D, Porter B, et al. Radiolabeled-antibody therapy of B-cell lymphoma with autologous bone marrow support. N Engl J Med. 1993;329(17):1219–24.
Knox SJ, Goris ML, Trisler K, Negrin R, Davis T, Liles TM, Grillo-López A, Chinn P, Varns C, Ning SC, Fowler S, Deb N, Becker M, Marquez C, Levy R. Yttrium-90-labeled anti-CD20 monoclonal antibody therapy of recurrent B-cell lymphoma. Clin Cancer Res. 1996;2(3):457–70.
Goldberg DM. Epratuzumab in the therapy of oncological and immunological diseases. Expert Rev Anticancer Ther. 2006;6:1341–53.
Griffiths GL, Govindan SV, Sharkey RM, et al. 90Y-DOTA-hLL2: an agent for radioimmunotherapy of non-Hodgkin’s lymphoma. J Nucl Med. 2003;44:77–84.
Lindén O, Hindorf C, Cavallin-Ståhl E, Wegener WA, Goldenberg DM, Horne H, Ohlsson T, Stenberg L, Strand SE, Tennvall J. Dose-fractionated radioimmunotherapy in non-Hodgkin’s lymphoma using DOTA-conjugated, 90Y-radiolabeled, humanized anti-CD22 monoclonal antibody, epratuzumab. Clin Cancer Res. 2005;11(14):5215–22.
Morschhauser F, Kraeber-Bodéré F, Wegener WA, Harousseau JL, Petillon MO, Huglo D, Trümper LH, Meller J, Pfreundschuh M, Kirsch CM, Naumann R, Kropp J, Horne H, Teoh N, Le Gouill S, Bodet-Milin C, Chatal JF, Goldenberg DM. High rates of durable responses with anti-CD22 fractionated radioimmunotherapy: results of a multicenter, phase I/II study in non-Hodgkin’s lymphoma. J Clin Oncol. 2010;28(23):3709–16. Epub 2010 Jul 12.
Kraeber-Bodere F, Pallardy A, Le Gouill, S, Maisonneuve H, Lamy T, Bouabdalla K, Milpied N, Jardel H, Deconinck E, Morineau N, Foussard C, Brion A, Gressin R, Tournilhac O, Gyan E, Moreau A, Berthou C, Dreyfus F, Bodet-Milin C, Cazeau AL, Garin E, Vuillez JP, Campion L, Moreau P, Wegener WA, Goldenberg DM, Soubeyran P. Consolidation anti-CD22 fractionated radioimmunotherapy with 90Y-epratuzumab tetraxetan following R-CHOP in elderly DLBCL patients: a Lysa phase II prospective trial. Presented at the 54th ASH Annual Meeting and Exposition, Atlanta, GA, 8–11 December 2012.
Mattes MJ, Sharkey RM, Karacay H, Czuczman MS, Goldenberg DM. Therapy of advanced B-lymphoma xenografts with a combination of 90Y-anti-CD22 IgG (epratuzumab) and unlabeled anti-CD20 IgG (veltuzumab). Clin Cancer Res. 2008;14(19):6154–60.
Tomblyn MB, Witzig TE, Himelstein AL, Elstrom R, Kio EA, Sharkey RM, Rojo J, Wegener W, Goldenberg DM. Combination therapy targeting two different antigens with anti-CD22 radioimmunotherapy and anti-CD20 immunotherapy in non-Hodgkin lymphoma (NHL): phase I results. Presented at the 54th ASH Annual Meeting and Exposition, Atlanta, GA, 8–11 December 2012.
Sharkey RM, Brenner A, Burton J, Hajjar G, Toder SP, Alavi A, Matthies A, Tsai DE, Schuster SJ, Stadtmauer EA, Czuczman MS, Lamonica D, Kraeber-Bodere F, Mahe B, Chatal JF, Rogatko A, Mardirrosian G, Goldenberg DM. Radioimmunotherapy of non-Hodgkin’s lymphoma with 90Y-DOTA humanized anti-CD22 IgG (90Y-Epratuzumab): do tumor targeting and dosimetry predict therapeutic response? J Nucl Med. 2003;44(12):2000–18.
Pagel JM, Pantelias A, Hedin N, Wilbur S, Saganic L, Lin Y, Axworthy D, Hamlin DK, Wilbur DS, Gopal AK, Press OW. Evaluation of CD20, CD22, and HLA-DR targeting for radioimmunotherapy of B-cell lymphomas. Cancer Res. 2007;67(12):5921–8.
FitzGerald DJ, Wayne AS, Kreitman RJ, Pastan I. Treatment of hematologic malignancies with immunotoxins and antibody-drug conjugates. Cancer Res. 2011;71(20):6300–9.
Kantarjian H, Thomas D, Jorgensen J, Jabbour E, Kebriaei P, Rytting M, York S, Ravandi F, Kwari M, Faderl S, Rios MB, Cortes J, Fayad L, Tarnai R, Wang SA, Champlin R, Advani A, O’Brien S. Inotuzumab ozogamicin, an anti-CD22-calecheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: a phase 2 study. Lancet Oncol. 2012;13:403–11.
Goy A, et al. Inotuzumab ozogamicin (INO, CMC-544) in patients with indolent B-cell NHL refractory to rituximab, R plus. In: 11th International conference of malignant lymphoma, Abstract 069. Lugano, Switzerland, 15–18 June 2011.
Ricart AD. Antibody-drug conjugates of calicheamicin derivative: gemtuzumab ozogamicin and inotuzumabozogamicin. Clin Cancer Res. 2011;17(20):6417–27.
Pfizer. An open-label, randomized, phase 3 study of inotuzumab ozogamicin administered in combination with rituximab compared to defined investigator’s choice therapy in subjects with relapsed or refractory CD22-positive aggressive non-hodgkin lymphoma who are not candidates for intensive high-dose chemotherapy. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 [cited 2013 Feb 08]. http://clinicaltrials.gov/show/NCT01232556. NLM Identifier: NCT01232556.
Pfizer. An open-label, randomized phase 3 study of inotuzumab ozogamicin compared to a defined investigator’s choice in adult patients with relapsed or refractory CD22-positive acute lymphoblastic leukemia (ALL). In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 [cited 2013 Feb 08]. http://clinicaltrials.gov/show/NCT01564784. NLM Identifier: NCT01564784.
DiJoseph JF, Dougher MM, Evans DY, Zhou BB, Damle NK. Preclinical anti-tumor activity of antibody-targeted chemotherapy with CMC-544 (inotuzumab ozogamicin), a CD22-specific immunoconjugate of calicheamicin, compared with non-targeted combination chemotherapy with CVP or CHOP. Cancer Chemother Pharmacol. 2011;67(4):741–9. Epub 2010 Jun 3.
Dijoseph JF, Dougher MM, Armellino DC, Evans DY, Damle NK. Therapeutic potential of CD22-specific antibody-targeted chemotherapy using inotuzumab ozogamicin (CMC-544) for the treatment of acute lymphoblastic leukemia. Leukemia. 2007;21(11):2240–5. Epub 2007 Jul 26.
Kato J, O’Donnell RT, Abuhay M, Tuscano JM. Efficacy and toxicity of a CD22-targeted antibody-saporin conjugate in a xenograft model of a non-Hodgkin’s lymphoma. OncoImmunology. 2012;1(9):1–7.
Kato J, et al. Efficacy of a CD22-targeted antibody-saporin conjugate in a xenograft model of precursor-B cell acute lymphoblastic leukemia. Leuk Res. 2012. http://dx.doi.org/10.1016/j.leukres.2012.09.010.
Salvatore G, Beers R, Margulies I, Kreitman RJ, Pastan I. Improved cytotoxic activity toward cell lines and fresh leukemia cells of a mutant anti-CD22 immunotoxin obtained by antibody phage display. Clin Cancer Res. 2002;8(4):995–1002.
Mansfield E, Pastan I, Fitzgerald DJ. Characterization of FRB4-pseudomonas exotoxin A immunotoxins targeted to CD22 on B-cell malignancies. Bioconjug Chem. 1996;7(5):557–63.
Kreitman RJ, Tallman MS, Robak T, Coutre S, Wilson WH, Stetler-Stevenson M, Fitzgerald DJ, Lechleider R, Pastan I. Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J Clin Oncol. 2012;30(15):1822–8. Epub 2012 Feb 21.
Wayne AS, Bhojwani D, Silverman LB, Richards K, Stetler-Stevenson M, Shah NN, Jeha S, Pui CH, Buzoianu M, FitzGerald DJ, Kreitman RJ, Ibrahim R, Pastan I. A novel anti-CD22 immunotoxin, moxetumomab pasudotox: phase I study in pediatric acute lymphoblastic leukemia (ALL). Abstract 248, presented at the 53rd American Society of Hematology Annual Meeting and Exposition, 10–13 December 2011, San Diego, CA.
Wei H, Xiang L, Wayne AS, Chertov O, FitzGerald DJ, Bera TK, Pastan I. Immunotoxin resistance via reversible methylation of the DPH4 promoter is a unique survival strategy. Proc Natl Acad Sci USA. 2012;109(18):6898–903. Epub 2012 Apr 16.
Kreitman RJ, Wilson WH, Bergeron K, Raggio M, Stetler-Stevenson M, FitzGerald DJ, Pastan I. Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. N Engl J Med. 2001;345(4):241–7.
Pastan I, Onda M, Weldon J, Fitzgerald D, Kreitman R. Immunotoxins with decreased immunogenicity and improved activity. Leuk Lymphoma. 2011;52(Suppl 2):87–90. Epub 2011 Apr 19.
Wayne AS, Kreitman RJ, Findley HW, Lew G, Delbrook C, Steinberg SM, Stetler-Stevenson M, Fitzgerald DJ, Pastan I. Anti-CD22 immunotoxin RFB4(dsFv)-PE38 (BL22) for CD22-positive hematologic malignancies of childhood: preclinical studies and phase I clinical trial. Clin Cancer Res. 2010;16(6):1894–903.
Sharkey RM, Govindan SV, Cardillo TM, Goldenberg DM. Epratuzumab-SN-38: a new antibody-drug conjugate for the therapy of hematologic malignancies. Mol Cancer Ther. 2012;11(1):224–34. Epub 2011 Oct 28.
Polson AG, Williams M, Gray AM, Fuji RN, Poon KA, McBride J, Raab H, Januario T, Go M, Lau J, Yu SF, Du C, Fuh F, Tan C, Wu Y, Liang WC, Prabhu S, Stephan JP, Hongo JA, Dere RC, Deng R, Cullen M, de Tute R, Bennett F, Rawstron A, Jack A, Ebens A. Anti-CD22-MCC-DM1: an antibody-drug conjugate with a stable linker for the treatment of non-Hodgkin’s lymphoma. Leukemia. 2010;24(9):1566–73. Epub 2010 July 1.
Adair JR, Howard PW, Hartley JA, Williams DG, Chester KA. Antibody-drug conjugates - a perfect synergy. Expert Opin Biol Ther. 2012;12(9):1191–206.
Polson AG, Ho WY, Ramakrishnan V. Investigational antibody-drug conjugates for hematological malignancies. Expert Opin Investig Drugs. 2011;20(1):75–85.
Tateno H, Li H, Schur MJ, Bovin N, Crocker PR, Wakarchuk WW, Paulson JC. Distinct endocytic mechanisms of CD22 (Siglec-2) and Siglec-F reflect roles in cell signaling and innate immunity. Mol Cell Biol. 2007;27(16):5699–710. Epub 2007 June 11.
Collins BE, Blixt O, Han S, Duong B, Li H, Nathan JK, Bovin N, Paulson JC. High-affinity ligand probes of CD22 overcome the threshold set by cis ligands to allow for binding, endocytosis, and killing of B cells. J Immunol. 2006;177(5):2994–3003.
Chen WC, Sigal DS, Saven A, Paulson JC. Targeting B lymphoma with nanoparticles bearing glycan ligands of CD22. Leuk Lymphoma. 2012;53(2):208–10. Epub 2011 Aug 24.
Chen WC, Completo GC, Sigal DS, Crocker PR, Saven A, Paulson JC. In vivo targeting of B-cell lymphoma with glycan ligands of CD22. Blood. 2010;115(23):4778–86. Epub 2010 Feb 24. Erratum in: Blood. 2011;117(20):5551.
O’Reilly MK, Tian H, Paulson JC. CD22 is a recycling receptor that can shuttle cargo between the cell surface and endosomal compartments of B cells. J Immunol. 2011;186(3):1554–63. Epub 2010 Dec 22.
Sapra P, Allen TM. Internalizing antibodies are necessary for improved therapeutic efficacy of antibody-targeted liposomal drugs. Cancer Res. 2002;62(24):7190–4.
O’Donnell RT, Martin SM, Ma YP, Zamboni WC, Tuscano JM. Development and characterization of CD22-targeted pegylatedliposomal doxorubicin (IL-PLD). Invest New Drugs. 2010;28(3):260–7.
Tuscano JM, Martin SM, Ma Y, Zamboni W, O’Donnell RT. Efficacy, biodistribution, and pharmacokinetics of CD22-targeted pegylated liposomal doxorubicin in a B-cell non-Hodgkin’s lymphoma xenograft mouse model. Clin Cancer Res. 2010;16(10):2760–8.
Tedder TF, Tuscano JM, Sato S, Kehrl JH. CD22, a B lymphocyte-specific adhesion molecule that regulates antigen receptor signaling. Annu Rev Immunol. 1997;15:481–504.
O’Donnell RT, Martin SM, Ma YP, Zamboni WC, Tuscano JM. Dose, timing, schedule, and the choice of targeted epitope alter the efficacy of anti-CD22 immunotherapy in mice bearing human lymphoma xenografts. Cancer Imunol Immunother. 2009;58(12):2051–8.
Poe JC, Fujimoto Y, Hasegawa M, Haas KM, Miller AS, Sanford IG, Bock CB, Fujimoto M, Tedder TF. CD22 regulates B lymphocyte function in vivo through both ligand-dependent and ligand-independent mechanisms. Nat Immunol. 2004;5(10):1078–87. Epub 2004 Sep 19.
McKoy JM, Angelotta C, Bennett CL, Tallman MS, Wadleigh M, Evens AM, Kuzel TM, Trifilio SM, Raisch DW, Kell J, DeAngelo DJ, Giles FJ. Gemtuzumab ozogamicin-associated sinusoidal obstructive syndrome (SOS): an overview from the research on adverse drug events and reports (RADAR) project. Leuk Res. 2007;31(5):599–604. Epub 2006 Sep 7.
Goozner M. FDA increases focus on postmarketing studies. J Natl Cancer Inst. 2010;102(17):1302–4. Epub 2010 Aug 25.
Tanimoto T, Tsubokura M, Mori J, Pietrek M, Ono S, Kami M. Differences in drug approval processes of 3 regulatory agencies: a case study of gemtuzumab ozogamicin. Invest New Drugs. 2013;31(2):473–8.
Mitka M. Oversight of fast-track drug approval by FDA stuck in low gear, critics say. JAMA. 2010;304(16):1773–5.
Bezalel S, Asher I, Elbirt D, Sthoeger ZM. Novel biological treatments for systemic lupus erythematosus: current and future modalities. Isr Med Assoc J. 2012;14(8):508–14.
Toba K, Hanawa H, Fuse I, Sakaue M, Watanabe K, Uesugi Y, Higuchi W, Takahashi M, Aizawa Y. Difference in CD22 molecules in human B cells and basophils. Exp Hematol. 2002;30(3):205–11.
Mott RT, Ait-Ghezala G, Town T, Mori T, Vendrame M, Zeng J, Ehrhart J, Mullan M, Tan J. Neuronal expression of CD22: novel mechanism for inhibiting microglial proinflammatory cytokine production. Glia. 2004;46(4):369–79.
Tuscano JM, Kato J, Pearson D, Xiong C, Newell L, Ma Y, Gandara DR, O’Donnell RT. CD22 Antigen is broadly expressed on lung cancer cells and is a target for antibody-based therapy. Cancer Res. 2012;72(21):5556–65.
Acknowledgements
No sources of funding were used to prepare this manuscript. The authors have no conflicts of interest that are directly relevant to the content of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sullivan-Chang, L., O’Donnell, R.T. & Tuscano, J.M. Targeting CD22 in B-cell Malignancies: Current Status and Clinical Outlook. BioDrugs 27, 293–304 (2013). https://doi.org/10.1007/s40259-013-0016-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-013-0016-7