Abstract
Background and Objective
Gastric/gastrointestinal cancers are associated with high mortality worldwide. G-protein coupled receptor (GPCR) superfamily members such as gastrin/cholecystokinin-B receptor (CCK-BR) are involved in progression of gastric tumors, thus CCK-BR is considered as a potential target for immunotherapy. However, production of functional monoclonal antibodies (mAbs) against GPCR seems to be very challenging, in part due to its integration in cell membranes and inaccessibility for selection. To tackle this problem, we implemented phage display technology and a solution-phase biopanning (SPB) scheme for production of mAbs specific to the native conformation of CCK-BR.
Methods
To perform the SPB process, we utilized a synthetic biotinylated peptide corresponding to the second extracellular loop (ECL2) of CCK-BR and a semi-synthetic phage antibody library. After enzyme-linked immunosorbent assay (ELISA) screening, the CCK-BR specificity of the selected single-chain variable fragments (scFvs) were further examined using immunoblotting, whole-cell ELISA, and flow cytometry assays.
Results
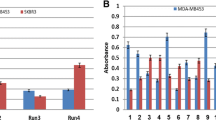
After performing four rounds of selection, we identified nine antibody clones which showed positive reactivity with the CCK-BR peptide in an ELISA assay. Of these, eight clones were unique scFv antibodies and one was a VL single domain antibody. Specificity analysis of the selected scFvs revealed that five of the selected scFvs recognized a denatured form of CCK-BR, while the majority of the selected scFvs were able to recognize the native conformation of CCK-BR on the surface of human gastric adenocarcinoma cells and cervical carcinoma HeLa cells.
Conclusion
For the first time, we report on the establishment of a diverse panel of scFv antibody fragments that are specific to the native conformation of CCK-BR. Based on these results, we suggest the selected scFv antibody fragments as potential agents for diagnosis, imaging, targeting, and/or immunotherapy of cancers that overexpress CCK-BR.






Similar content being viewed by others
References
Sasako M, Inoue M, Lin JT, et al. Gastric cancer working group report. Jpn J Clin Oncol. 2010;40(Suppl. 1):i28–37.
Hartgrink HH, Jansen EP, van Grieken NC, et al. Gastric cancer. Lancet. 2009;374:477–90.
Yamashita K, Sakuramoto S, Watanabe M. Genomic and epigenetic profiles of gastric cancer: potential diagnostic and therapeutic applications. Surg Today. 2011;41:24–38.
Arkenau HT. Gastric cancer in the era of molecularly targeted agents: current drug development strategies. J Cancer Res Clin Oncol. 2009;135:855–66.
Burkitt MD, Varro A, Pritchard DM. Importance of gastrin in the pathogenesis and treatment of gastric tumors. World J Gastroenterol. 2009;15:1–16.
Polk DB, Peek RM Jr. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10:403–14.
McCaig C, Duval C, Hemers E, et al. The role of matrix metalloproteinase-7 in redefining the gastric microenvironment in response to Helicobacter pylori. Gastroenterology. 2006;130:1754–63.
Varro A, Noble PJ, Pritchard DM, et al. Helicobacter pylori induces plasminogen activator inhibitor 2 in gastric epithelial cells through nuclear factor-kappaB and RhoA: implications for invasion and apoptosis. Cancer Res. 2004;64:1695–702.
Smith AM, Watson SA, Caplin M, et al. Gastric carcinoid expresses the gastrin autocrine pathway. Br J Surg. 1998;85:1285–9.
Stubbs M, Khan K, Watson SA, et al. Endocytosis of anti-CCK-B/gastrin receptor antibody and effect on hepatoma cell lines. J Histochem Cytochem. 2002;50:1213–7.
Watson SA, Clarke PA, Smith AM, et al. Expression of CCKB/gastrin receptor isoforms in gastro-intestinal tumour cells. Int J Cancer. 1998;77:572–7.
Varro A, Noble PJ, Wroblewski LE, et al. Gastrin-cholecystokinin(B) receptor expression in AGS cells is associated with direct inhibition and indirect stimulation of cell proliferation via paracrine activation of the epidermal growth factor receptor. Gut. 2002;50:827–33.
Barderas R, Shochat S, Timmerman P, et al. Designing antibodies for the inhibition of gastrin activity in tumoral cell lines. Int J Cancer. 2008;122:2351–9.
Gilliam AD, Watson SA, Henwood M, et al. A phase II study of G17DT in gastric carcinoma. Eur J Surg Oncol. 2004;30:536–43.
Ajani JA, Hecht JR, Ho L, et al. An open-label, multinational, multicenter study of G17DT vaccination combined with cisplatin and 5-fluorouracil in patients with untreated, advanced gastric or gastroesophageal cancer: the GC4 study. Cancer. 2006;106(9):1908–16.
McWilliams DF, Grimes S, Watson SA. Antibodies raised against the extracellular tail of the CCKB/gastrin receptor inhibit gastrin-stimulated signalling. Regul Pept. 2001;99:157–61.
Watson SA, Clarke PA, Morris TM, et al. Antiserum raised against an epitope of the cholecystokinin B/gastrin receptor inhibits hepatic invasion of a human colon tumor. Cancer Res. 2000;60:5902–7.
Hancock WW. Chemokines and transplant immunobiology. J Am Soc Nephrol. 2002;13:821–4.
Brett BT, Smith SC, Bouvier CV, et al. Phase II study of anti-gastrin-17 antibodies, raised to G17DT, in advanced pancreatic cancer. J Clin Oncol. 2002;20:4225–31.
Reichert JM. Marketed therapeutic antibodies compendium. MAbs. 2012;4:413–5.
Stockwin LH, Holmes S. Antibodies as therapeutic agents: vive la renaissance! Expert Opin Biol Ther. 2003;3:1133–52.
Hong H, Kim S. Antibody engineering. Biotechnol Bioprocess Eng. 2002;7:150–4.
Kim SJ, Park Y, Hong HJ. Antibody engineering for the development of therapeutic antibodies. Mol Cells. 2005;20:17–29.
Mondon P, Dubreuil O, Bouayadi K, et al. Human antibody libraries: a race to engineer and explore a larger diversity. Front Biosci. 2008;13:1117–29.
Sergeeva A, Kolonin MG, Molldrem JJ, et al. Display technologies: application for the discovery of drug and gene delivery agents. Adv Drug Deliv Rev. 2006;58:1622–54.
Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228:1315–7.
Bradbury AR, Marks JD. Antibodies from phage antibody libraries. J Immunol Methods. 2004;290:29–49.
Hoet RM, Cohen EH, Kent RB, et al. Generation of high-affinity human antibodies by combining donor-derived and synthetic complementarity-determining-region diversity. Nat Biotechnol. 2005;23:344–8.
Soderlind E, Strandberg L, Jirholt P, et al. Recombining germline-derived CDR sequences for creating diverse single-framework antibody libraries. Nat Biotechnol. 2000;18:852–6.
O’Connell D, Becerril B, Roy-Burman A, et al. Phage versus phagemid libraries for generation of human monoclonal antibodies. J Mol Biol. 2002;321:49–56.
Knappik A, Ge L, Honegger A, et al. Fully synthetic human combinatorial antibody libraries (HuCAL) based on modular consensus frameworks and CDRs randomized with trinucleotides. J Mol Biol. 2000;296:57–86.
Tohidkia MR, Barar J, Asadi F, et al. Molecular considerations for development of phage antibody libraries. J Drug Target. 2012;20:195–208.
Benhar I. Design of synthetic antibody libraries. Expert Opin Biol Ther. 2007;7:763–79.
Nelson AL. Antibody fragments: hope and hype. MAbs. 2010;2:77–83.
Majidi J, Barar J, Baradaran B, et al. Target therapy of cancer: implementation of monoclonal antibodies and nanobodies. Hum Antibodies. 2009;18:81–100.
Ben-Kasus T, Schechter B, Sela M, et al. Cancer therapeutic antibodies come of age: targeting minimal residual disease. Mol Oncol. 2007;1:42–54.
Sanz L, Blanco B, Alvarez-Vallina L. Antibodies and gene therapy: teaching old ‘magic bullets’ new tricks. Trends Immunol. 2004;25:85–91.
Weiner LM, Murray JC, Shuptrine CW. Antibody-based immunotherapy of cancer. Cell. 2012;148:1081–4.
Binyamin L, Borghaei H, Weiner LM. Cancer therapy with engineered monoclonal antibodies. Update Cancer Ther. 2006;1:147–57.
Beckman RA, Weiner LM, Davis HM. Antibody constructs in cancer therapy: protein engineering strategies to improve exposure in solid tumors. Cancer. 2007;109:170–9.
Adams GP, Schier R. Generating improved single-chain Fv molecules for tumor targeting. J Immunol Methods. 1999;231:249–60.
Smith J, Kontermann RE, Embleton J, et al. Antibody phage display technologies with special reference to angiogenesis. FASEB J. 2005;19:331–41.
Peeters MC, van Westen GJ, Li Q, et al. Importance of the extracellular loops in G protein-coupled receptors for ligand recognition and receptor activation. Trends Pharmacol Sci. 2011;32:35–42.
de Haard HJ, van Neer N, Reurs A, et al. A large non-immunized human Fab fragment phage library that permits rapid isolation and kinetic analysis of high affinity antibodies. J Biol Chem. 1999;274:18218–30.
Freson K, Van Geet C, Hoylaerts M, et al. Anti-VPAC1 antibodies and theire uses. In: WO Patent WO/2009/000,894; 2008.
Huang L, Sato AK, Sachdeva M, et al. Discovery of human antibodies against the C5aR target using phage display technology. J Mol Recognit. 2005;18:327–33.
Hawlisch H, Frank R, Hennecke M, et al. Site-directed C3a receptor antibodies from phage display libraries. J Immunol. 1998;160:2947–58.
Hoogenboom HR, Lutgerink JT, Pelsers MM, et al. Selection-dominant and nonaccessible epitopes on cell-surface receptors revealed by cell-panning with a large phage antibody library. Eur J Biochem. 1999;260:774–84.
Sui J, Bai J, St Clair Tallarico A, et al. Identification of CD4 and transferrin receptor antibodies by CXCR4 antibody-guided Pathfinder selection. Eur J Biochem. 2003;270:4497–506.
Mirzabekov T, Kontos H, Farzan M, et al. Paramagnetic proteoliposomes containing a pure, native, and oriented seven-transmembrane segment protein, CCR5. Nat Biotechnol. 2000;18:649–54.
Henderikx P, Kandilogiannaki M, Petrarca C, et al. Human single-chain Fv antibodies to MUC1 core peptide selected from phage display libraries recognize unique epitopes and predominantly bind adenocarcinoma. Cancer Res. 1998;58:4324–32.
Hawkins RE, Russell SJ, Winter G. Selection of phage antibodies by binding affinity: mimicking affinity maturation. J Mol Biol. 1992;226:889–96.
Emadi S, Barkhordarian H, Wang MS, et al. Isolation of a human single chain antibody fragment against oligomeric alpha-synuclein that inhibits aggregation and prevents alpha-synuclein-induced toxicity. J Mol Biol. 2007;368:1132–44.
Marks JD, Hoogenboom HR, Bonnert TP, et al. By-passing immunization. Human antibodies from V-gene libraries displayed on phage. J Mol Biol. 1991;222:581–97.
Sambrook J, Russell DW. Molecular cloning: a laboratory manual. 3rd ed. Cold Spring Harbor (NY): CSHL press; 2001.
Konthur Z, Wilde J. Evaluation of recombinant antibodies on protein microarrays applying the multiple spotting technique. Antibody Eng 2010; 447–60.
Retter I, Althaus HH, Münch R, et al. VBASE2, an integrative V gene database. Nucleic Acids Res. 2005;33:D671–4.
Kipriyanov SM, Moldenhauer G, Little M. High level production of soluble single chain antibodies in small-scale Escherichia coli cultures. J Immunol Methods. 1997;200:69–77.
Schmiedl A, Breitling F, Winter CH, et al. Effects of unpaired cysteines on yield, solubility and activity of different recombinant antibody constructs expressed in E. coli. J Immunol Methods. 2000;242:101–14.
Hutchings CJ, Koglin M, Marshall FH. Therapeutic antibodies directed at G protein-coupled receptors. MAbs. 2010;2:594–606.
Gupta A, Decaillot FM, Gomes I, et al. Conformation state-sensitive antibodies to G-protein-coupled receptors. J Biol Chem. 2007;282:5116–24.
Sidhu SS, editor. Phage display in biotechnology and drug discovery. Boca Raton (FL): CRC Press; 2005.
Hust M, Steinwand M, Al-Halabi L, et al. Improved microtitre plate production of single chain Fv fragments in Escherichia coli. N Biotechnol. 2009;25:424–8.
Goffinet M, Chinestra P, Lajoie-Mazenc I, et al. Identification of a GTP-bound Rho specific scFv molecular sensor by phage display selection. BMC Biotechnol. 2008;8:34.
Jensen KB, Larsen M, Pedersen JS, et al. Functional improvement of antibody fragments using a novel phage coat protein III fusion system. Biochem Biophys Res Commun. 2002;298:566–73.
Wang X, Campoli M, Ko E, et al. Enhancement of scFv fragment reactivity with target antigens in binding assays following mixing with anti-tag monoclonal antibodies. J Immunol Methods. 2004;294:23–35.
Wu S, Ke A, Doudna JA. A fast and efficient procedure to produce scFvs specific for large macromolecular complexes. J Immunol Methods. 2007;318:95–101.
Barderas R, Shochat S, Martinez-Torrecuadrada J, et al. A fast mutagenesis procedure to recover soluble and functional scFvs containing amber stop codons from synthetic and semisynthetic antibody libraries. J Immunol Methods. 2006;312:182–9.
Lebesgue D, Wallukat G, Mijares A, et al. An agonist-like monoclonal antibody against the human beta2-adrenoceptor. Eur J Pharmacol. 1998;348:123–33.
Teufel M, Pompejus M, Humbel B, et al. Properties of bacteriorhodopsin derivatives constructed by insertion of an exogenous epitope into extra-membrane loops. EMBO J. 1993;12:3399–408.
Verdot L, Bertin B, Guilloteau D, et al. Characterization of pharmacologically active anti-peptide antibodies directed against the first and second extracellular loops of the serotonin 5-HT1A receptor. J Neurochem. 1995;65:319–28.
Zhang Y, Pool C, Sadler K, et al. Selection of active scFv to G-protein-coupled receptor CCR5 using surface antigen-mimicking peptides. Biochemistry. 2004;43:12575–84.
Hall BL, Boroughs J, Kobrin BJ. A novel tumor-specific human single-chain Fv selected from an active specific immunotherapy phage display library. Immunotechnology. 1998;4:127–40.
Schwab C, Bosshard HR. Caveats for the use of surface-adsorbed protein antigen to test the specificity of antibodies. J Immunol Methods. 1992;147:125–34.
Adey NB, Mataragnon AH, Rider JE, et al. Characterization of phage that bind plastic from phage-displayed random peptide libraries. Gene. 1995;156:27–31.
Butler JE, Ni L, Nessler R, et al. The physical and functional behavior of capture antibodies adsorbed on polystyrene. J Immunol Methods. 1992;150:77–90.
Acknowledgments
This work was supported by the Digestive Disease Research Center (DDRC) at Tehran University of Medical Sciences and the Research Center for Pharmaceutical Nanotechnology (RCPN) at Tabriz University of Medical Sciences. The authors are grateful to Mr. Abolfazl Barzegari, Dr. Hossein Zareh and Dr. Safar Farajnia (Tabriz University of Medical Sciences) and Dr. Masoumeh Rajabi Bazli (Shaid Behesti University of Medical Sciences) for their useful comments.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Tohidkia, M.R., Asadi, F., Barar, J. et al. Selection of Potential Therapeutic Human Single-Chain Fv Antibodies against Cholecystokinin-B/Gastrin Receptor by Phage Display Technology. BioDrugs 27, 55–67 (2013). https://doi.org/10.1007/s40259-012-0007-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-012-0007-0