Abstract
Introduction
Psoriatic arthritis (PsA) is a chronic inflammatory arthritis that occurs in people affected by the autoimmune disease psoriasis. The cost effectiveness of secukinumab in PsA has not been evaluated in Germany.
Objective
The purpose of this study was to conduct a cost-utility analysis of secukinumab in three adult populations with active PsA in Germany: biologic naïve without moderate or severe plaque psoriasis, biologic naïve with moderate or severe plaque psoriasis, and biologic experienced. Comparators included other disease-modifying antirheumatic drugs (DMARDs), including biosimilar versions as well as standard of care.
Methods
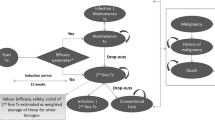
The analysis took the viewpoint of the German statutory health insurance. We adapted a decision analytic semi-Markov model to evaluate the cost effectiveness of secukinumab over a lifetime horizon. Treatment response was assessed based on PsA Response Criteria at 12 weeks. Nonresponders or patients discontinuing the initial-line DMARD were allowed to switch to subsequent-line DMARDs. Model input parameters (Psoriasis Area Severity Index, Health Assessment Questionnaire (HAQ), withdrawal rates, costs, and resource use) were collected from clinical trials, published literature, and official reports. Health benefits were expressed as quality-adjusted life-years. An annual discount rate of 3% was applied to costs and benefits. The robustness of the study findings was evaluated via sensitivity analyses.
Results
In the biologic-naïve population without moderate or severe plaque psoriasis, secukinumab 150 mg either strictly dominated other DMARDs (certolizumab pegol, golimumab, and ustekinumab) or yielded favorable incremental cost-effectiveness ratios (ICERs) (vs. etanercept, adalimumab, and infliximab). In the biologic-naïve population with concomitant moderate to severe plaque psoriasis and in the biologic-experienced population, secukinumab 300 mg was more effective and had a lower ICER than other DMARDs, thus leading to extended dominance. Deterministic sensitivity analyses indicated that the results were most sensitive to the discount rate for costs and health outcomes as well as the HAQ score as an input to utility values.
Conclusions
Secukinumab appears to be cost effective compared with other DMARDs for the treatment of active PsA in biologic-naïve and biologic-experienced populations in Germany.
Similar content being viewed by others
Data Availability
The datasets used for the cost-effectiveness analysis are not publicly available for proprietary reasons.
References
Gossec L, Smolen JS, Ramiro S, de Wit M, Cutolo M, Dougados M, Emery P, Landewé R, Oliver S, Aletaha D, Betteridge N, Braun J, Burmester G, Cañete JD, Damjanov N, FitzGerald O, Haglund E, Helliwell P, Kvien TK, Lories R, Luger T, Maccarone M, Marzo-Ortega H, McGonagle D, McInnes IB, Olivieri I, Pavelka K, Schett G, Sieper J, van den Bosch F, Veale DJ, Wollenhaupt J, Zink A, van der Heijde D. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75(3):499–510.
Elsevier Health Analytics. Übersichtsbericht der Ergebnisse: Versorgungsforschung Psoriasis (Pso), Psoriasisarthritis (PsA) und axiale Spondyloarthritis (AxSpA) in Deutschland; eine retrospektive Analyse auf Routinedaten; 2017.
Cosentyx. Summary of product characteristics. Last updated: 25/10/2018. https://www.ema.europa.eu/documents/product-information/cosentyx-epar-product-information_en.pdf (accessed 10 Aug 2019).
McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, et al. Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;386(9999):1137–46.
Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, van der Heijde D, et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Engl J Med. 2015;373(14):1329–39.
Gemeinsamer Bundesausschuss. Tragende Gründe zum Beschluss des Gemeinsamen Bundesausschusses über eine Änderung der Arzneimittel-Richtlinie (AM-RL): Anlage XII - Beschlüsse über die Nutzenbewertung von Arzneimitteln mit neuen Wirkstoffen nach § 35a SGB V—Secukinumab (neues Anwendungsgebiet). Gemeinsamer Bundesausschuss; 2. Juni 2016.
Gemeinsamer Bundesausschuss. Tragende Gründe zum Beschluss des Gemeinsamen Bundesausschusses über eine Änderung der Arzneimittel-Richtlinie (AM-RL): Anlage XII – Beschlüsse über die Nutzenbewertung von Arzneimitteln mit neuen Wirkstoffen nach § 35a SGB V—Ixekizumab (neues Anwendungsgebiet: Psoriasis-Arthritis). Gemeinsamer Bundesausschuss; 16. August 2018.
Buchanan V, Sullivan W, Graham C, Miles L, Jugl SM, Gunda P, Halliday A, Kirkham B. Cost effectiveness of secukinumab for the treatment of active psoriatic arthritis in the UK. Pharmacoeconomics. 2018;36(7):867–78.
Goeree R, Chiva-Razavi S, Gunda P, Graham CN, Miles L, Nikoglou E, Jugl SM, Gladman DD. Cost-effectiveness analysis of secukinumab for the treatment of active psoriatic arthritis: a Canadian perspective. J Med Econ. 2018;21(2):163–73.
Purmonen T, Puolakka K, Bhattacharyya D, Jain M, Martikainen J. Cost-effectiveness analysis of secukinumab versus other biologics and apremilast in the treatment of active psoriatic arthritis: a Finnish perspective. Cost Eff Resour Alloc. 2018;16(16):56.
McInnes I, Nash P, Ritchlin C, et al. THU0437 Secukinumab for the treatment of psoriatic arthritis: comparative effectiveness results versus licensed biologics and apremilast from a network meta-analysis. Ann Rheum Dis. 2016;75:348–9.
Spitzenverband Bund der Krankenkassen. GKV-Arzneimittel-Schnellinformation für Deutschland GKV-Arzneimittel-Schnellinformation für Deutschland. Berlin: GKV-Spitzenverband; 2018.
NICE Multiple Technology Appraisal—Certolizumab pegol and secukinumab for treating active psoriatic arthritis following inadequate response to disease modifying anti-rheumatic drugs, London; 2017. https://www.nice.org.uk/guidance/ta445/documents/html-content-3 (accessed 10 Aug 2019).
The Dental and Pharmaceutical Benefits Agency (TLV): Cosentyx ingår i högkostnadsskyddet med begränsning, Stockholm; 2016. https://www.tlv.se/beslut/beslut-lakemedel/begransad-subvention/arkiv/2016-05-31-cosentyx-ingar-i-hogkostnadsskyddet-med-begransning.html (accessed 10 Aug 2019).
McInnes IB, Nash P, Ritchlin C, Choy EH, Kanters S, Thom H, Gandhi K, Pricop L, Jugl SM. Secukinumab for psoriatic arthritis: comparative effectiveness versus licensed biologics/apremilast: a network meta-analysis. J Comp Eff Res. 2018;7(11):1107–23.
Mease PJ, Goffe BS, Metz J, VanderStoep A, Finck B, Burge DJ. Etanercept in the treatment of psoriatic arthritis and psoriasis: a randomised trial. Lancet. 2000;356(9227):385–90.
Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, Porter AC, Tugwell P, Moher D, Bouter LM. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;15(7):10.
Rodgers M, Epstein D, Bojke L, Yang H, Craig D, Fonseca T, Myers L, Bruce I, Chalmers R, Bujkiewicz S, Lai M, Cooper N, Abrams K, Spiegelhalter D, Sutton A, Sculpher M, Woolacott N. Etanercept, infliximab and adalimumab for the treatment of psoriatic arthritis: a systematic review and economic evaluation. Health Technol Assess. 2011;15(10):i–xxi, 1–329.
Cawson MR, Mitchell SA, Knight C, Wildey H, Spurden D, Bird A, Orme ME. Systematic review, network meta-analysis and economic evaluation of biological therapy for the management of active psoriatic arthritis. BMC Musculoskelet Disord. 2014;20(15):26.
Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen. General methods. Version 5.0. Köln: IQWiG; 2017.
Lauer-Fischer GmbH. Lauer-Taxe Arzneimittelpreise. http://www.lauer-fischer.de (accessed 1 June 2018).
Rychlik R. Kosten der Unterversorgung mit Arzneimitteln in Deutschland. Februar: Gutachten für den Verband Forschender Arzneimittelhersteller e.V; 2008.
Berger K, Ehlken B, Kugland B, Augustin M. Cost-of-illness in patients with moderate and severe chronic psoriasis vulgaris in Germany. J Dtsch Dermatol Ges. 2005;3(7):511–8.
Kassenärztliche Bundesvereinigung. Einheitlicher. Bewertungsmaßstab. Stand: 1. Quartal 2018. Berlin; 2018.
Robert Koch Institut. Bericht zum Krebsgeschehen in Deutschland 2016, Berlin; 2016.
Statistisches Bundesamt (Destatis). Krankheitskosten 2015 in Deutschland nach Diagnosen, Einrichtungen, Alter und Geschlecht. Statistisches Bundesamt (Destatis); 2017.
Dolan P. Modeling valuations for EuroQol health states. Medical Care; 1997. p. 1095–1108.
Wong K, Gladman DD, Husted J, Long JA, Farewell VT. Mortality studies in psoriatic arthritis: results from a single outpatient clinic I Causes and risk of death. Arthritis Rheumatol. 1997;40(10):1868–72.
Abuabara K, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the UK. Br J Dermatol. 2010;163(3):586–92.
Statistisches Bundesamt (Destatis). Sterbetafel (Periodensterbetafel): Deutschland, Jahre, Geschlecht, Vollendetes Alter. Statistisches Bundesamt (Destatis); 2018.
Prosser LA, Neumann PJ, Sanders GD, Siegel JE. Reporting cost-effectiveness analyses. In: Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG, editors. Cost-effectiveness in health and medicine. Oxford: Oxford University Press; 2016.
Fagerli KM, Lie E, van der Heijde D, Heiberg MS, Kalstad S, Rødevand E, Mikkelsen K, Lexberg AS, Kvien TK. Switching between TNF inhibitors in psoriatic arthritis: data from the NOR-DMARD study. Ann Rheum Dis. 2013;72(11):1840–4.
Glintborg B, Ostergaard M, Krogh NS, Andersen MD, Tarp U, Loft AG, Lindegaard HM, Holland-Fischer M, Nordin H, Jensen DV, Olsen CH, Hetland ML. Clinical response, drug survival, and predictors thereof among 548 patients with psoriatic arthritis who switched tumor necrosis factor α inhibitor therapy: results from the Danish Nationwide DANBIO Registry. Arthritis Rheumatol. 2013;65(5):1213–23.
Harrold LR, Stolshek BS, Rebello S, Collier DH, Mutebi A, Wade SW, Malley W, Greenberg JD, Etzel CJ. Rebound in measures of disease activity and symptoms in Corrona Registry patients with psoriatic arthritis who discontinue tumor necrosis factor inhibitor therapy after achieving low disease activity. J Rheumatol. 2018;45(1):78–82.
Strand V, Elaine Husni M, Betts KA, Song Y, Singh R, Griffith J, Beppu M, Zhao J, Ganguli A. Network meta-analysis and cost per responder of targeted Immunomodulators in the treatment of active psoriatic arthritis. BMC Rheumatol. 2018;12(2):3.
Author information
Authors and Affiliations
Contributions
AG was involved in model conceptualization and adaptation and wrote the first draft of the manuscript. DO was involved in model conceptualization and commented on the draft version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
DO is the founder and director of WifOR, which was contracted by Novartis Deutschland GmbH to undertake this work. AG is a consultant for WifOR.
Funding
This work was contracted by Novartis Deutschland GmbH.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gandjour, A., Ostwald, D.A. Cost Effectiveness of Secukinumab Versus Other Biologics and Apremilast in the Treatment of Active Psoriatic Arthritis in Germany. Appl Health Econ Health Policy 18, 109–125 (2020). https://doi.org/10.1007/s40258-019-00523-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-019-00523-1