Abstract
Background
Nearly 20,000 people were diagnosed with multi-drug and rifampicin-resistant tuberculosis (MDR/RR-TB) in South Africa in 2015, yet only one-half of the patients who start treatment are expected to have a successful outcome. There is increasing evidence of the effectiveness and safety of new drug regimens containing bedaquiline for MDR/RR-TB; however, whether they are affordable for high-burden, limited-resource settings is uncertain.
Objective
Our objective was to determine the incremental cost effectiveness of a bedaquiline-based regimen for MDR/RR-TB treatment in South Africa compared with the standard kanamycin-based regimen.
Methods
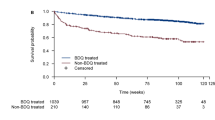
We established a Markov model for ambulatory treatment of MDR/RR-TB in a high-HIV prevalence setting, parameterized using clinical outcomes from the South African National TB Programme (SA NTP) before (2012–2014) and after (2015–2016) bedaquiline roll-out. The effectiveness of treatment was evaluated in disability-adjusted life-years (DALYs). Ingredient costs from the provider’s perspective were collected in 2016 South African Rand and converted to $US, including bedaquiline at $US675.23 per 6-month treatment course. Culture conversion rates were derived from the phase IIb trial of bedaquiline, and disability adjustments were adapted from published literature. Costs and effectiveness were discounted at 3%.
Results
For non-bedaquiline regimens, the total expected cost over the 10-year time horizon for a patient with MDR/RR-TB was $US4439 with disability-adjusted survival of 5.1 years. Replacing capreomycin with bedaquiline in patients who failed MDR/RR-TB treatment and required treatment for extensively drug-resistant (XDR-TB) resulted in cost savings ($US4356; 1.8% less) and similar effectiveness (0.02 DALYs averted). As a result, the standard regimen (no bedaquiline) was dominated. Replacing kanamycin with bedaquiline to provide all patients with MDR/RR-TB access to bedaquiline cost $US4647 (4.3% more) and averted 0.17 DALYs compared with the no bedaquiline regimen. The incremental cost-effectiveness ratio was $US1242/DALY averted.
Conclusion
Markov modelling indicates providing bedaquiline for all patients with MDR/RR-TB could increase the 24-month treatment success rate in South Africa from 56.3% using the current regimen to 60.6%, at a cost $US2.6 million over a 10-year horizon, less than 1% of the estimated $US425 million SA NTP annual budget.


Similar content being viewed by others
References
World Health Organization. Global Tuberculosis Report. Geneva: WHO. 2016. http://www.who.int/tb/publications/global_report/en/.
Diacon AH, Pym AS, Grobusch MP, de los Rios JM, Gotuzzo E, Vasilyeva I, Leimane V, Andries K, Bakare N, De Marez T, Haxaire-Theeuwes M, Lounis N, Meyvisch P, De Paepe E, van Heeswijk RPG, Dannemann B. Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N Engl J Med. 2014;371:723–32. doi:10.1056/NEJMoa1313865.
World Health Organization. Report of the Guideline Development Group Meeting on the use of bedaquiline in the treatment of multidrug-resistant tuberculosis: a review of available evidence. Geneva: WHO. 2017. http://apps.who.int/iris/bitstream/10665/254712/1/WHO-HTM-TB-2017.01-eng.pdf?ua=1.
Borisov SE, Dheda K, Enwerem M, Leyet RR, Ambrosio LD, Centis R, Denholm J, Douglas P, Duarte R, Esmail A, Gualano G, Jonsson J, Kunst H, Lau JS, Mastrapa BL, Lazaro J, Troya T, Manga S, Manika K, Montaner PG, Mullerpattan J. Effectiveness and safety of bedaquiline-containing regimens in the treatment of MDR- and XDR-TB: a multicentre study. Eur Respir J. 2017;49:1700387. doi:10.1183/13993003.00387-2017.
Tiberi S, Scardigli A, Centis R, Ambrosio LD, Spanevello A, Miguel A, Mun M, Visca D, Zumla A, Battista G, Caminero JA. Classifying new anti-tuberculosis drugs: rationale and future perspectives. Int J Infect Dis. 2017;56:181–4. doi:10.1016/j.ijid.2016.10.026.
Caminero JA, Scardigli A. Classification of antituberculosis drugs: a new proposal based on the most recent evidence. Eur Respir J. 2015; pp 887–893. doi:10.1183/13993003.00432-2015.
Pontali E, Sotgiu G, D’Ambrosio L, Centis R, Migliori GB. Bedaquiline and multidrug-resistant tuberculosis: a systematic and critical analysis of the evidence. Eur Respir J. 2016;47:394–402. doi:10.1183/13993003.01891-2015.
World Health Organization. The use of bedaquiline in the treatment of multi-drug resistant tuberculosis: Interim policy guidance. Geneva: WHO. 2013. http://apps.who.int/iris/bitstream/10665/84879/1/9789241505482_eng.pdf.
Wolfson LJ, Gibbert J, Wirth D, Diel R. Cost-effectiveness of incorporating bedaquiline into a treatment regimen for multidrug-resistant/extensively drug-resistant tuberculosis in Germany. Eur Respir J. 2015;46:1826–9. doi:10.1183/13993003.00811-2015.
H.Y. Park, H.M. Ku, H.S. Sohn, H.S. Seo, H. Yung Lee, K. Hwa Lim, J.W. Kwon, Cost-effectiveness of Bedaquiline for the Treatment of Multidrug-resistant Tuberculosis in the Republic of Korea, Clin. Ther. 38 (2016) 655–667.e2. doi:10.1016/j.clinthera.2016.01.023.
Wolfson LJ, Walker A, Hettle R, Lu X, Kambili C, Murungi A, Knerer G. Cost-effectiveness of adding bedaquiline to drug regimens for the treatment of multidrug-resistant tuberculosis in the UK. PLoS One. 2015;10:1–20. doi:10.1371/journal.pone.0120763.
Lu X, Smare C, Kambili C, El Khoury AC, Wolfson LJ. Health outcomes of bedaquiline in the treatment of multidrug-resistant tuberculosis in selected high burden countries. BMC Health Serv Res. 2017;17:87. doi:10.1186/s12913-016-1931-3.
Directorate Drug-Resistant TB management of drug-resistant. TB: policy guidelines. Pretoria: National Department of Health. 2013.
Directorate Drug-Resistant TB TB & HIV, Introduction of new drugs, drug regimens and management for drug-resistant TB in South Africa: Policy framework, 1.1. Pretoria: National Department of Health. 2015.
Berhanu RH, Schnippel KL, Mohr E, Hirasen K, Evans D, Rosen S, Sanne I. Early outcomes of decentralized care for rifampicin-resistant tuberculosis in Johannesburg, South Africa: an Observational Cohort Study. PLoS One. 2016;11:e0164974. doi:10.1371/journal.pone.0164974.
Cox HS, Hughes J, Daniels J, Azevedo V, McDermid C, Poolman M, Boulle A, Goemaere E, Van Cutsem G. Community-based treatment of drug-resistant tuberculosis in Khayelitsha, South Africa. Int J Tuberc Lung Dis. 2014;18:441–8. doi:10.5588/ijtld.13.0742.
Schnippel KL, Shearer K, Evans D, Berhanu R, Dlamini S, Ndjeka N. Predictors of mortality and treatment success during treatment for rifampicin-resistant tuberculosis within the South African National TB Programme, 2009 to 2011: a cohort analysis of the national case register. Int J Infect Dis. 2015;39(2015):89–94. doi:10.1016/j.ijid.2015.09.002.
Schnippel K, Firnhaber C, Ndjeka N, Conradie F, Page-Shipp L, Berhanu R, Sinanovic E. Persistently high early mortality despite rapid diagnostics for drug-resistant tuberculosis cases in South Africa. Int J Tuberc Lung Dis. 2017;21(10):1106–11. doi:10.5588/ijtld.17.0202.
Cox H, Dickson-hall L, Ndjeka N, Van Hoog A, Grant A, Cobelens F, Stevens W, Nicol M. Delays and loss to follow-up before treatment of drug-resistant tuberculosis following implementation of Xpert MTB/RIF in South Africa: a retrospective cohort study. 2017; pp 1–19. doi:10.5061/dryad.051h0.
World Health Organization. Global Health Observatory data repository. Life tables by country: South Africa. Geneva: WHO. 2013. http://apps.who.int/gho/data/?theme=main&vid=61540. Accessed 28 March 2016.
USForex Inc. dba OFX, Historical Exchange Rates (2016). https://www.ofx.com/en-us/forex-news/historical-exchange-rates/. Accessed 5 March 2017.
Statistics South Africa. Consumer Price Index, January 2016. Pretoria: Statistics South Africa. http://www.statssa.gov.za/publications/P0141/P0141January2016.pdf.
National Health Laboratory Service. State Price List (2015). http://www.nhls.ac.za.
South Africa National Department of Health Essential Drug Programme, Master Procurement Catalogue. 2016. http://www.health.gov.za/index.php/component/phocadownload/category/196. Accessed 25 March 2016.
South Africa National Department of Health. UPFS Fee Schedule for Full Paying Patients. 2015. https://www.westerncape.gov.za/general-publication/western-cape-government-hospital-tariffs-overview?toc_page=4.
Cox HS, Ramma L, Wilkinson L, Azevedo V, Sinanovic E. Cost per patient of treatment for rifampicin-resistant tuberculosis in a community-based program in Khayelitsha, South Africa. Trop Med Int Health. 2015;20:1337–45. doi:10.1111/tmi.12544.
Sinanovic E, Ramma L, Vassall A, Azevedo V, Wilkinson L, Ndjeka N, Mccarthy K, Churchyard GJ, Cox H. Impact of reduced hospitalisation on the cost of treatment for drug-resistant tuberculosis in South Africa. Int J Tuberc Lung Dis. 2015;19:172–8. doi:10.5588/ijtld.14.0421.
Massyn N, Peer N, Padarath A, Barron P, Day C. District Health Barometer. 2015. http://www.hst.org.za/sites/default/files/DHB_annual_district_1Sep2015.xlsx.
Salomon JA, Vos T, Hogan DR, Gagnon M, Naghavi M, Mokdad A, Begum N. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380:2129–43. doi:10.1016/S0140-6736(12)61680-8.
Krahn M, Kuntz KM, Meltzer DO, Owens DK, Russell LB, Siegel JE, Ganiats TG. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–103. doi:10.1001/jama.2016.12195.
Ndjeka N, Conradie F, Schnippel KL, Hughes J, Bantubani N, Ferreira H, Maartens G, Mametja D, Meintjes G, Padanilam X, Variava E, Pym AS, Pillay Y. Treatment of drug-resistant tuberculosis with bedaquiline in a high HIV prevalence setting: an interim cohort analysis. Int J Tuberc Lung Dis. 2015;19:979–85. doi:10.5588/ijtld.14.0944.
Codecasa LR, Toumi M, D’Ausilio A, Aiello A, Damele F, Termini R, Uglietti A, Hettle R, Graziano G, De Lorenzo S. Cost-effectiveness of bedaquiline in MDR and XDR tuberculosis in Italy. J Mark Access Health Policy. 2017;5:1283105. doi:10.1080/20016689.2017.1283105.
Pooran A, Pieterson E, Davids M, Theron G, Dheda K. What is the cost of diagnosis and management of drug resistant tuberculosis in South Africa? PLoS One. 2013;8:e54587. doi:10.1371/journal.pone.0054587.
Pietersen E, Ignatius E, Streicher EM, Mastrapa B, Padanilam X, Pooran A, Badri M, Lesosky M, van Helden P, Sirgel SA, Warren R, Dheda K. Long-term outcomes of patients with extensively drug-resistant tuberculosis in South Africa: a cohort study. Lancet. 2014;383:1230–9. doi:10.1016/S0140-6736(13)62675-6.
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, Augustovski F, Briggs AH. Consolidated health economic evaluation reporting standards (CHEERS) statement. Value Health. 2013;16:e1–5. doi:10.1016/j.jval.2013.02.010.
Ndjeka N, Conradie F, Schnippel KL, Hughes J, Bantubani N, Ferreira H, Maartens G. Treatment of drug-resistant tuberculosis with bedaquiline in a high HIV prevalence setting: an interim cohort analysis. Int J Tuberc Lung Dis. 2015;19:979–85. doi:10.5588/ijtld.14.0944.
Flanagan W, Mcintosh CN, Le Petit C, Berthelot J-M. Multiplicative model for combining individual condition scores. Popul Health Metr. 2006;4:1–8. doi:10.1186/1478-7954-4-13.
Acknowledgements
This work would not have been possible without the dedication of the clinicians and specialists of the Bedaquiline Clinical Access Programme Clinical Advisory Committee. Special thanks also to each of the RR TB units rolling out bedaquiline and to all of the patients at these units. This analysis is of work led by the South African National Department of Health and collected through the SA NTP, with thanks to Y Pillay, LD Mametja, S Dlamini, and P Richards. Thank you to R Laubscher from the Medical Research Council for matching to the vital statistics register.
Author information
Authors and Affiliations
Contributions
KS conceptualized the analysis, built and parameterized the cost-effectiveness model, drafted the manuscript and incorporated all author comments and revisions. CF and ES advised on the analysis design and methods, reviewed the analysis results, and reviewed and critically revised the manuscript. FC and NN assisted in providing access to the data, supported interpretation and understanding of the model parameters, and reviewed the manuscript. All authors approved the manuscript submitted for publication.
Corresponding author
Ethics declarations
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflict of Interest
KS, CF, FC, NN and ES have no conflicts of interest. NN is an official at the South African National Department of Health and therefore has responsibility for establishing and implementing national treatment guidelines for drug-resistant TB.
Ethics Statement
All procedures performed in the study involving human participants (i.e. analysis of transition probabilities from the EDRweb case register) were in accordance with the 1964 Helsinki declaration and its later amendments and the ethical standards the Human Research Ethics Committee of the University of Cape Town (#490/2015, July 2015) and the Human Research Ethics Committee of the University of Witwatersrand (#M150340, March 2015). For this type of secondary retrospective analysis of routinely collected data, formal consent is not required. A data-sharing agreement was submitted to the Research Information Monitoring, Evaluation and Surveillance Directorate within the South African National Department of Health for access to the EDRweb. Methods and results are presented according to the CHEERS checklist for economic evaluations [35].
Rights and permissions
About this article
Cite this article
Schnippel, K., Firnhaber, C., Conradie, F. et al. Incremental Cost Effectiveness of Bedaquiline for the Treatment of Rifampicin-Resistant Tuberculosis in South Africa: Model-Based Analysis. Appl Health Econ Health Policy 16, 43–54 (2018). https://doi.org/10.1007/s40258-017-0352-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40258-017-0352-8