Abstract
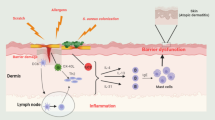
Atopic dermatitis is one of the most common chronic inflammatory skin diseases. It usually begins in childhood, has a considerable impact on patients’ quality of life, and incurs substantial healthcare costs. The standard-of-care treatments for patients with moderate to severe disease are very limited and have variable and typically insufficient efficacy and many side effects, some of which are quite serious. However, over the last decade, considerable advances in our understanding of the pathogenesis of atopic dermatitis have paved the way for a number of new treatments. Most notable are the drugs that target the Th2-polarized immune system, which is thought to play a key role in many of the signs and symptoms characteristic of this disease. In this article, we briefly review the pathophysiology of atopic dermatitis, while noting that each patient’s disease phenotype is likely due to a unique interplay of several disease-specific dysregulated pathways. Lastly, we cover emerging therapies for atopic dermatitis, focusing on those that target specific components of the immune system, which are altered in atopic dermatitis. The hope is that these new biologics or small-molecule antagonists, which have high specificity for their target molecules, will decrease the undesirable side effects caused by off-target effects commonly observed with current immunosuppressive agents that are characterized by broad biological actions.


Similar content being viewed by others
References
Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242(1):233–46.
Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis–part II: immune cell subsets and therapeutic concepts. J Allergy Clin Immunol. 2011;127(6):1420–32.
Baker BS. The role of microorganisms in atopic dermatitis. Clin Exp Immunol. 2006;144(1):1–9.
Silverberg JI, Hanifin J, Simpson EL. Climatic factors are associated with childhood eczema prevalence in the United States. J Invest Dermatol. 2013;133(7):1752–9.
Finlay AY. Quality of life in atopic dermatitis. J Am Acad Dermatol. 2001;45(1 Suppl):S64–6.
Ellis CN, Drake LA, Prendergast MM, Abramovits W, Boguniewicz M, Daniel CR, et al. Cost of atopic dermatitis and eczema in the United States. J Am Acad Dermatol. 2002;46(3):361–70.
Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116–32.
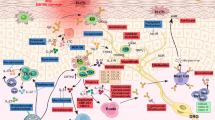
Mansouri Y, Guttman-Yassky E. Immune pathways in atopic dermatitis, and definition of biomarkers through broad and targeted therapeutics. J Clin Med. 2015;4(5):858–73.
Sidbury R, Davis DM, Cohen DE, Cordoro KM, Berger TG, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71(2):327–49.
Roekevisch E, Spuls PI, Kuester D, Limpens J, Schmitt J. Efficacy and safety of systemic treatments for moderate-to-severe atopic dermatitis: a systematic review. J Allergy Clin Immunol. 2014;133(2):429–38.
Bae JM, Choi YY, Park CO, Chung KY, Lee KH. Efficacy of allergen-specific immunotherapy for atopic dermatitis: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2013;132(1):110–7.
Elias PM, Steinhoff M. “Outside-to-inside” (and now back to “outside”) pathogenic mechanisms in atopic dermatitis. J Invest Dermatol. 2008;128(5):1067–70.
Hvid M, Vestergaard C, Kemp K, Christensen GB, Deleuran B, Deleuran M. IL-25 in atopic dermatitis: a possible link between inflammation and skin barrier dysfunction? J Invest Dermatol. 2011;131(1):150–7.
Oyoshi MK, He R, Kumar L, Yoon J, Geha RS. Cellular and molecular mechanisms in atopic dermatitis. Adv Immunol. 2009;102:135–226.
Chapman MD, Wunschmann S, Pomes A. Proteases as Th2 adjuvants. Curr Allergy Asthma Rep. 2007;7(5):363–7.
Hammad H, Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015;43(1):29–40.
Leung DY, Guttman-Yassky E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J Allergy Clin Immunol. 2014;134(4):769–79.
Eigenmann PA, Sicherer SH, Borkowski TA, Cohen BA, Sampson HA. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics. 1998;101(3):E8.
Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38(4):441–6.
Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365(14):1315–27.
Winge MC, Bilcha KD, Lieden A, Shibeshi D, Sandilands A, Wahlgren CF, et al. Novel filaggrin mutation but no other loss-of-function variants found in Ethiopian patients with atopic dermatitis. Br J Dermatol. 2011;165(5):1074–80.
Margolis DJ, Gupta J, Apter AJ, Hoffstad O, Papadopoulos M, Rebbeck TR, et al. Exome sequencing of filaggrin and related genes in African-American children with atopic dermatitis. J Invest Dermatol. 2014;134(8):2272–4.
Henderson J, Northstone K, Lee SP, Liao H, Zhao Y, Pembrey M, et al. The burden of disease associated with filaggrin mutations: a population-based, longitudinal birth cohort study. J Allergy Clin Immunol. 2008;121(4):872–7 e9.
Tokura Y. Extrinsic and intrinsic types of atopic dermatitis. J Dermatol Sci. 2010;58(1):1–7.
Akdis CA, Akdis M. Immunological differences between intrinsic and extrinsic types of atopic dermatitis. Clin Exp Allergy. 2003;33(12):1618–21.
Suarez-Farinas M, Dhingra N, Gittler J, Shemer A, Cardinale I, de Guzman Strong C, et al. Intrinsic atopic dermatitis shows similar TH2 and higher TH17 immune activation compared with extrinsic atopic dermatitis. J Allergy Clin Immunol. 2013;132(2):361–70.
Kabashima-Kubo R, Nakamura M, Sakabe J, Sugita K, Hino R, Mori T, et al. A group of atopic dermatitis without IgE elevation or barrier impairment shows a high Th1 frequency: possible immunological state of the intrinsic type. J Dermatol Sci. 2012;67(1):37–43.
Kuo IH, Yoshida T, De Benedetto A, Beck LA. The cutaneous innate immune response in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131(2):266–78.
Leyden JJ, Marples RR, Kligman AM. Staphylococcus aureus in the lesions of atopic dermatitis. Br J Dermatol. 1974;90(5):525–30.
Hasannejad H, Takahashi R, Kimishima M, Hayakawa K, Shiohara T. Selective impairment of Toll-like receptor 2-mediated proinflammatory cytokine production by monocytes from patients with atopic dermatitis. J Allergy Clin Immunol. 2007;120(1):69–75.
Niebuhr M, Langnickel J, Sigel S, Werfel T. Dysregulation of CD36 upon TLR-2 stimulation in monocytes from patients with atopic dermatitis and the TLR2 R753Q polymorphism. Exp Dermatol. 2010;19(8):e296–8.
Mrabet-Dahbi S, Dalpke AH, Niebuhr M, Frey M, Draing C, Brand S, et al. The Toll-like receptor 2 R753Q mutation modifies cytokine production and Toll-like receptor expression in atopic dermatitis. J Allergy Clin Immunol. 2008;121(4):1013–9.
Ahmad-Nejad P, Mrabet-Dahbi S, Breuer K, Klotz M, Werfel T, Herz U, et al. The toll-like receptor 2 R753Q polymorphism defines a subgroup of patients with atopic dermatitis having severe phenotype. The J Allergy Clin Immunol. 2004;113(3):565–7.
Oh DY, Schumann RR, Hamann L, Neumann K, Worm M, Heine G. Association of the toll-like receptor 2 A-16934T promoter polymorphism with severe atopic dermatitis. Allergy. 2009;64(11):1608–15.
Potaczek DP, Nastalek M, Okumura K, Wojas-Pelc A, Undas A, Nishiyama C. An association of TLR2-16934A>T polymorphism and severity/phenotype of atopic dermatitis. J Eur Acad Dermatol Venereol. 2011;25(6):715–21.
Sumikawa Y, Asada H, Hoshino K, Azukizawa H, Katayama I, Akira S, et al. Induction of beta-defensin 3 in keratinocytes stimulated by bacterial lipopeptides through toll-like receptor 2. Microbes Infect. 2006;8(6):1513–21.
Baroni A, Orlando M, Donnarumma G, Farro P, Iovene MR, Tufano MA, et al. Toll-like receptor 2 (TLR2) mediates intracellular signalling in human keratinocytes in response to Malassezia furfur. Arch Dermatol Res. 2006;297(7):280–8.
Yuki T, Yoshida H, Akazawa Y, Komiya A, Sugiyama Y, Inoue S. Activation of TLR2 enhances tight junction barrier in epidermal keratinocytes. J Immunol. 2011;187(6):3230–7.
Kuo IH, Carpenter-Mendini A, Yoshida T, McGirt LY, Ivanov AI, Barnes KC, et al. Activation of epidermal toll-like receptor 2 enhances tight junction function: implications for atopic dermatitis and skin barrier repair. J Invest Dermatol. 2013;133(4):988–98.
Le TA, Takai T, Vu AT, Kinoshita H, Chen X, Ikeda S, et al. Flagellin induces the expression of thymic stromal lymphopoietin in human keratinocytes via toll-like receptor 5. Int Arch Allergy Immunol. 2011;155(1):31–7.
Vu AT, Baba T, Chen X, Le TA, Kinoshita H, Xie Y, et al. Staphylococcus aureus membrane and diacylated lipopeptide induce thymic stromal lymphopoietin in keratinocytes through the Toll-like receptor 2-Toll-like receptor 6 pathway. J Allergy Clin Immunol. 2010;126(5):985–93, 93 e1–3.
Kinoshita H, Takai T, Le TA, Kamijo S, Wang XL, Ushio H, et al. Cytokine milieu modulates release of thymic stromal lymphopoietin from human keratinocytes stimulated with double-stranded RNA. J Allergy Clin Immunol. 2009;123(1):179–86.
Koyasu S, Moro K. Type 2 innate immune responses and the natural helper cell. Immunology. 2011;132(4):475–81.
Lee GR, Flavell RA. Transgenic mice which overproduce Th2 cytokines develop spontaneous atopic dermatitis and asthma. Int Immunol. 2004;16(8):1155–60.
Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell SR, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5(7):752–60.
Gittler JK, Shemer A, Suarez-Farinas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQ, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130(6):1344–54.
Jeong CW, Ahn KS, Rho NK, Park YD, Lee DY, Lee JH, et al. Differential in vivo cytokine mRNA expression in lesional skin of intrinsic vs. extrinsic atopic dermatitis patients using semiquantitative RT-PCR. Clin Exp Allergy. 2003;33(12):1717–24.
Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. 2006;117(2):411–7.
Bilsborough J, Leung DY, Maurer M, Howell M, Boguniewicz M, Yao L, et al. IL-31 is associated with cutaneous lymphocyte antigen-positive skin homing T cells in patients with atopic dermatitis. J Allergy Clin Immunol. 2006;117(2):418–25.
Lund S, Walford HH, Doherty TA. Type 2 innate lymphoid cells in allergic disease. Curr Immunol Rev. 2013;9(4):214–21.
Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol. 2015;15(5):271–82.
Zhang Y, Zhou B. Functions of thymic stromal lymphopoietin in immunity and disease. Immunol Res. 2012;52(3):211–23.
Gandhi NA, Bennett BL, Graham NM, Pirozzi G, Stahl N, Yancopoulos GD. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2016;15(1):35–50.
Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT. 2013;2(3):e24137.
McKenzie GJ, Fallon PG, Emson CL, Grencis RK, McKenzie AN. Simultaneous disruption of interleukin (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses. J Exp Med. 1999;189(10):1565–72.
Bacharier LB, Geha RS. Molecular mechanisms of IgE regulation. J Allergy Clin Immunol. 2000;105(2 Pt 2):S547–58.
Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, DeBenedetto A, et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2009;124(3 Suppl 2):R7–12.
Kim BE, Leung DY, Boguniewicz M, Howell MD. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin Immunol. 2008;126(3):332–7.
Albanesi C, Fairchild HR, Madonna S, Scarponi C, De Pita O, Leung DY, et al. IL-4 and IL-13 negatively regulate TNF-alpha- and IFN-gamma-induced beta-defensin expression through STAT-6, suppressor of cytokine signaling (SOCS)-1, and SOCS-3. J Immunol. 2007;179(2):984–92.
Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, Hall CF, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171(6):3262–9.
Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347(15):1151–60.
Howell MD, Wollenberg A, Gallo RL, Flaig M, Streib JE, Wong C, et al. Cathelicidin deficiency predisposes to eczema herpeticum. J Allergy Clin Immunol. 2006;117(4):836–41.
Brauweiler AM, Goleva E, Leung DY. Th2 cytokines increase Staphylococcus aureus alpha toxin-induced keratinocyte death through the signal transducer and activator of transcription 6 (STAT6). J Invest Dermatol. 2014;134(8):2114–21.
Kouro T, Takatsu K. IL-5- and eosinophil-mediated inflammation: from discovery to therapy. Int Immunol. 2009;21(12):1303–9.
Zhang Q, Putheti P, Zhou Q, Liu Q, Gao W. Structures and biological functions of IL-31 and IL-31 receptors. Cytokine Growth Factor Rev. 2008;19(5–6):347–56.
Lee CH, Hong CH, Yu WT, Chuang HY, Huang SK, Chen GS, et al. Mechanistic correlations between two itch biomarkers, cytokine interleukin-31 and neuropeptide beta-endorphin, via STAT3/calcium axis in atopic dermatitis. Br J Dermatol. 2012;167(4):794–803.
Raap U, Wichmann K, Bruder M, Stander S, Wedi B, Kapp A, et al. Correlation of IL-31 serum levels with severity of atopic dermatitis. J Allergy Clin Immunol. 2008;122(2):421–3.
Toda M, Leung DY, Molet S, Boguniewicz M, Taha R, Christodoulopoulos P, et al. Polarized in vivo expression of IL-11 and IL-17 between acute and chronic skin lesions. J Allergy Clin Immunol. 2003;111(4):875–81.
Guttman-Yassky E, Lowes MA, Fuentes-Duculan J, Zaba LC, Cardinale I, Nograles KE, et al. Low expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasis. J Immunol. 2008;181(10):7420–7.
Hamid Q, Boguniewicz M, Leung DY. Differential in situ cytokine gene expression in acute versus chronic atopic dermatitis. J Clin Invest. 1994;94(2):870–6.
Werfel T, Morita A, Grewe M, Renz H, Wahn U, Krutmann J, et al. Allergen specificity of skin-infiltrating T cells is not restricted to a type-2 cytokine pattern in chronic skin lesions of atopic dermatitis. J Invest Dermatol. 1996;107(6):871–6.
Spergel JM, Mizoguchi E, Oettgen H, Bhan AK, Geha RS. Roles of TH1 and TH2 cytokines in a murine model of allergic dermatitis. J Clin Invest. 1999;103(8):1103–11.
Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, Kikuchi T, et al. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol. 2009;123(6):1244–52 e2.
Czarnowicki T, Gonzalez J, Shemer A, Malajian D, Xu H, Zheng X, et al. Severe atopic dermatitis is characterized by selective expansion of circulating TH2/TC2 and TH22/TC22, but not TH17/TC17, cells within the skin-homing T-cell population. J Allergy Clin Immunol. 2015;136(1):104–15 e7.
Koga C, Kabashima K, Shiraishi N, Kobayashi M, Tokura Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J Invest Dermatol. 2008;128(11):2625–30.
Teraki Y, Sakurai A, Izaki S. IL-13/IL-22-coproducing T cells, a novel subset, are increased in atopic dermatitis. J Allergy Clin Immunol. 2013;132(4):971–4.
Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210(13):2939–50.
Oldhoff JM, Darsow U, Werfel T, Katzer K, Wulf A, Laifaoui J, et al. Anti-IL-5 recombinant humanized monoclonal antibody (mepolizumab) for the treatment of atopic dermatitis. Allergy. 2005;60(5):693–6.
Oldhoff JM, Darsow U, Werfel T, Bihari IC, Katzer K, Laifaoui J, et al. No effect of anti-interleukin-5 therapy (mepolizumab) on the atopy patch test in atopic dermatitis patients. Int Arch Allergy Immunol. 2006;141(3):290–4.
Thompson CA. Mepolizumab approved as add-on long-term therapy for severe asthma. Am J Health Syst Pharm. 2015;72(24):2125.
Borish LC, Nelson HS, Lanz MJ, Claussen L, Whitmore JB, Agosti JM, et al. Interleukin-4 receptor in moderate atopic asthma. A phase I/II randomized, placebo-controlled trial. Am J Respir Crit Care Med. 1999;160(6):1816–23.
Borish LC, Nelson HS, Corren J, Bensch G, Busse WW, Whitmore JB, et al. Efficacy of soluble IL-4 receptor for the treatment of adults with asthma. J Allergy Clin Immunol. 2001;107(6):963–70.
Hart TK, Blackburn MN, Brigham-Burke M, Dede K, Al-Mahdi N, Zia-Amirhosseini P, et al. Preclinical efficacy and safety of pascolizumab (SB 240683): a humanized anti-interleukin-4 antibody with therapeutic potential in asthma. Clin Exp Immunol. 2002;130(1):93–100.
Gauvreau GM, Boulet LP, Cockcroft DW, Fitzgerald JM, Carlsten C, Davis BE, et al. Effects of interleukin-13 blockade on allergen-induced airway responses in mild atopic asthma. Am J Respir Crit Care Med. 2011;183(8):1007–14.
Corren J, Busse W, Meltzer EO, Mansfield L, Bensch G, Fahrenholz J, et al. A randomized, controlled, phase 2 study of AMG 317, an IL-4Ralpha antagonist, in patients with asthma. Am J Respir Crit Care Med. 2010;181(8):788–96.
Wenzel S, Wilbraham D, Fuller R, Getz EB, Longphre M. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet. 2007;370(9596):1422–31.
Groves RW, Wilbraham D, Fuller R, Longphre M. Inhibition of IL-4 and IL-13 with an IL-4 mutein (Aeroderm) protects against flares in atopic eczema. J Invest Dermatol. 2007;127:S54.
Thaci D, Simpson EL, Beck LA, Bieber T, Blauvelt A, Papp K, et al. Efficacy and safety of dupilumab in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical treatments: a randomised, placebo-controlled, dose-ranging phase 2b trial. Lancet. 2016;387(10013):40–52. doi:10.1016/S0140-6736(15)00388-8.
Beck LA, Thaci D, Hamilton JD, Graham NM, Bieber T, Rocklin R, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371(2):130–9.
Hamilton JD, Suarez-Farinas M, Dhingra N, Cardinale I, Li X, Kostic A, et al. Dupilumab improves the molecular signature in skin of patients with moderate-to-severe atopic dermatitis. J Allergy Clin Immunol. 2014;134(6):1293–300.
Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368(26):2455–66.
Pauwels B, Jonstam K, Bachert C. Emerging biologics for the treatment of chronic rhinosinusitis. Expert Rev Clin Immunol. 2015;11(3):349–61.
Tavakolpour S, Tavakolpour V. Interleukin 4 inhibition as a potential therapeutic in pemphigus. Cytokine. 2016;77:189–95.
Hanania NA, Noonan M, Corren J, Korenblat P, Zheng Y, Fischer SK, et al. Lebrikizumab in moderate-to-severe asthma: pooled data from two randomised placebo-controlled studies. Thorax. 2015;70(8):748–56.
Herrick CA, MacLeod H, Glusac E, Tigelaar RE, Bottomly K. Th2 responses induced by epicutaneous or inhalational protein exposure are differentially dependent on IL-4. J Clin Invest. 2000;105(6):765–75.
Brightling CE, Chanez P, Leigh R, O’Byrne PM, Korn S, She D, et al. Efficacy and safety of tralokinumab in patients with severe uncontrolled asthma: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med. 2015;3(9):692–701.
Danese S, Rudzinski J, Brandt W, Dupas JL, Peyrin-Biroulet L, Bouhnik Y, et al. Tralokinumab for moderate-to-severe UC: a randomised, double-blind, placebo-controlled, phase IIa study. Gut. 2015;64(2):243–9.
Walsh GM. Tralokinumab, an anti-IL-13 mAb for the potential treatment of asthma and COPD. Curr Opin Investig Drugs. 2010;11(11):1305–12.
Murray LA, Zhang H, Oak SR, Coelho AL, Herath A, Flaherty KR, et al. Targeting interleukin-13 with tralokinumab attenuates lung fibrosis and epithelial damage in a humanized SCID idiopathic pulmonary fibrosis model. Am J Respir Cell Mol Biol. 2014;50(5):985–94.
Roman M, Madkan VK, Chiu MW. Profile of secukinumab in the treatment of psoriasis: current perspectives. Ther Clin Risk Manag. 2015;11:1767–77.
Ling Y, Puel A. IL-17 and infections. Actas Dermosifiliogr. 2014;105(Suppl 1):34–40.
Burmester GR, Durez P, Shestakova G, Genovese MC, Schulze-Koops H, Li Y, et al. Association of HLA-DRB1 alleles with clinical responses to the anti-interleukin-17A monoclonal antibody secukinumab in active rheumatoid arthritis. Rheumatology (Oxford). 2016;55(1):49–55.
Baeten D, Sieper J, Braun J, Baraliakos X, Dougados M, Emery P, et al. Secukinumab, an interleukin-17A inhibitor, in ankylosing spondylitis. N Engl J Med. 2015;373(26):2534–48.
Letko E, Yeh S, Foster CS, Pleyer U, Brigell M, Grosskreutz CL, et al. Efficacy and safety of intravenous secukinumab in noninfectious uveitis requiring steroid-sparing immunosuppressive therapy. Ophthalmology. 2015;122(5):939–48.
Mease PJ, McInnes IB, Kirkham B, Kavanaugh A, Rahman P, van der Heijde D, et al. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N Engl J Med. 2015;373(14):1329–39.
Fernandez O, Arnal-Garcia C, Arroyo-Gonzalez R, Brieva L, Calles-Hernandez MC, Casanova-Estruch B, et al. Review of the novelties presented at the 28th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) (III). Rev Neurol. 2013;57(7):317–29.
MacGlashan D. Loss of receptors and IgE in vivo during treatment with anti-IgE antibody. J Allergy Clin Immunol. 2004;114(6):1472–4.
Heil PM, Maurer D, Klein B, Hultsch T, Stingl G. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course—a randomized, placebo-controlled and double blind pilot study. J Dtsch Dermatol Ges. 2010;8(12):990–8.
Krathen RA, Hsu S. Failure of omalizumab for treatment of severe adult atopic dermatitis. J Am Acad Dermatol. 2005;53(2):338–40.
Vigo PG, Girgis KR, Pfuetze BL, Critchlow ME, Fisher J, Hussain I. Efficacy of anti-IgE therapy in patients with atopic dermatitis. J Am Acad Dermatol. 2006;55(1):168–70.
Kim DH, Park KY, Kim BJ, Kim MN, Mun SK. Anti-immunoglobulin E in the treatment of refractory atopic dermatitis. Clin Exp Dermatol. 2013;38(5):496–500.
Lane JE, Cheyney JM, Lane TN, Kent DE, Cohen DJ. Treatment of recalcitrant atopic dermatitis with omalizumab. J Am Acad Dermatol. 2006;54(1):68–72.
Arm JP, Bottoli I, Skerjanec A, Floch D, Groenewegen A, Maahs S, et al. Pharmacokinetics, pharmacodynamics and safety of QGE031 (ligelizumab), a novel high-affinity anti-IgE antibody, in atopic subjects. Clin Exp Allergy. 2014;44(11):1371–85.
Chu SY, Horton HM, Pong E, Leung IW, Chen H, Nguyen DH, et al. Reduction of total IgE by targeted coengagement of IgE B-cell receptor and FcgammaRIIb with Fc-engineered antibody. J Allergy Clin Immunol. 2012;129(4):1102–15.
O’Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311–28.
Levy LL, Urban J, King BA. Treatment of recalcitrant atopic dermatitis with the oral Janus kinase inhibitor tofacitinib citrate. J Am Acad Dermatol. 2015;73(3):395–9.
Lee EB, Fleischmann R, Hall S, Wilkinson B, Bradley JD, Gruben D, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;370(25):2377–86.
Keystone EC, Taylor PC, Drescher E, Schlichting DE, Beattie SD, Berclaz PY, et al. Safety and efficacy of baricitinib at 24 weeks in patients with rheumatoid arthritis who have had an inadequate response to methotrexate. Ann Rheum Dis. 2015;74(2):333–40.
Jabbari A, Dai Z, Xing L, Cerise JE, Ramot Y, Berkun Y, et al. Reversal of alopecia areata following treatment with the JAK1/2 inhibitor baricitinib. EBioMedicine. 2015;2(4):351–5.
Agusti-Mejias A, Messeguer F, Garcia R, Febrer I. Severe refractory atopic dermatitis in an adolescent patient successfully treated with ustekinumab. Ann Dermatol. 2013;25(3):368–70.
Samorano LP, Hanifin JM, Simpson EL, Leshem YA. Inadequate response to ustekinumab in atopic dermatitis—a report of two patients. J Eur Acad Dermatol Venereol. 2016;30(3):522–3.
Puya R, Alvarez-Lopez M, Velez A, Asuncion EC, Moreno JC. Treatment of severe refractory adult atopic dermatitis with ustekinumab. Int J Dermatol. 2012;51(1):115–6.
Kalb RE, Fiorentino DF, Lebwohl MG, Toole J, Poulin Y, Cohen AD, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the psoriasis longitudinal assessment and registry (PSOLAR). JAMA Dermatol. 2015;151(9):961–9.
Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133(2):448–60.
Nobbe S, Dziunycz P, Muhleisen B, Bilsborough J, Dillon SR, French LE, et al. IL-31 expression by inflammatory cells is preferentially elevated in atopic dermatitis. Acta Derm Venereol. 2012;92(1):24–8.
Nemoto O, Furue M, Nakagawa H, Shiramoto M, Hanada R, Matsuki S, et al. The first trial of CIM331, a humanized anti-human IL-31 receptor A antibody, for healthy volunteers and patients with atopic dermatitis to evaluate safety, tolerability and pharmacokinetics of a single dose in a randomised, double-blind, placebo-controlled study. Br J Dermatol. 2016;174(2):296–304. doi:10.1111/bjd.14207.
Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3(7):673–80.
Gauvreau GM, O’Byrne PM, Boulet LP, Wang Y, Cockcroft D, Bigler J, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014;370(22):2102–10.
Kanhere A, Hertweck A, Bhatia U, Gokmen MR, Perucha E, Jackson I, et al. T-bet and GATA3 orchestrate Th1 and Th2 differentiation through lineage-specific targeting of distal regulatory elements. Nat Commun. 2012;3:1268.
Grewe SR, Chan SC, Hanifin JM. Elevated leukocyte cyclic AMP-phosphodiesterase in atopic disease: a possible mechanism for cyclic AMP-agonist hyporesponsiveness. J Allergy Clin Immunol. 1982;70(6):452–7.
Gantner F, Tenor H, Gekeler V, Schudt C, Wendel A, Hatzelmann A. Phosphodiesterase profiles of highly purified human peripheral blood leukocyte populations from normal and atopic individuals: a comparative study. J Allergy Clin Immunol. 1997;100(4):527–35.
Samrao A, Berry TM, Goreshi R, Simpson EL. A pilot study of an oral phosphodiesterase inhibitor (apremilast) for atopic dermatitis in adults. Arch Dermatol. 2012;148(8):890–7.
Volf EM, Au SC, Dumont N, Scheinman P, Gottlieb AB. A phase 2, open-label, investigator-initiated study to evaluate the safety and efficacy of apremilast in subjects with recalcitrant allergic contact or atopic dermatitis. J Drugs Dermatol. 2012;11(3):341–6.
Schmidt BM, Kusma M, Feuring M, Timmer WE, Neuhauser M, Bethke T, et al. The phosphodiesterase 4 inhibitor roflumilast is effective in the treatment of allergic rhinitis. J Allergy Clin Immunol. 2001;108(4):530–6.
van Schalkwyk E, Strydom K, Williams Z, Venter L, Leichtl S, Schmid-Wirlitsch C, et al. Roflumilast, an oral, once-daily phosphodiesterase 4 inhibitor, attenuates allergen-induced asthmatic reactions. J Allergy Clin Immunol. 2005;116(2):292–8.
Stein GLF, Spelman L, Spellman MC, Hughes MH, Zane LT. A phase 2, randomized, controlled, dose-ranging study evaluating crisaborole topical ointment, 0.5% and 2% in adolescents with mild to moderate atopic dermatitis. J Drugs Dermatol. 2015;14(12):1394–9.
Murrell DF, Gebauer K, Spelman L, Zane LT. Crisaborole topical ointment, 2% in adults with atopic dermatitis: a phase 2a, vehicle-controlled, proof-of-concept study. J Drugs Dermatol. 2015;14(10):1108–12.
Tom WL, Van Syoc M, Chanda S, Zane LT. pharmacokinetic profile, safety, and tolerability of crisaborole topical ointment, 2% in adolescents with atopic dermatitis: an open-label phase 2a study. Pediatr Dermatol. 2016;33(2):150–9.
Zane LT, Kircik L, Call R, Tschen E, Draelos ZD, Chanda S, et al. Crisaborole topical ointment, 2% in patients ages 2 to 17 years with atopic dermatitis: a phase 1b, open-label, maximal-use systemic exposure study. Pediatr Dermatol. 2016. doi:10.1111/pde.12872.
Draelos ZD, Stein GLF, Murrell DF, Hughes MH, Zane LT. Post hoc analyses of the effect of crisaborole topical ointment, 2% on atopic dermatitis: associated pruritus from phase 1 and 2 clinical studies. J Drugs Dermatol. 2016;15(2):172–6.
Anacor Pharmaceuticals. Anacor Pharmaceuticals announces positive top-line results from two phase 3 pivotal studies of crisaborole topical ointment, 2% in patients with mild-to-moderate atopic dermatitis [media release]. 2015 Jul 13 [online]. http://investor.anacor.com/releasedetail.cfm?ReleaseID=921668. Accessed 10 Dec 2015.
Furue M, Kitahara Y, Akama H, Hojo S, Hayashi N, Nakagawa H, et al. Safety and efficacy of topical E6005, a phosphodiesterase 4 inhibitor, in Japanese adult patients with atopic dermatitis: results of a randomized, vehicle-controlled, multicenter clinical trial. J Dermatol. 2014;41(7):577–85.
Ohba F, Nomoto M, Hojo S, Akama H. Safety, tolerability and pharmacokinetics of a novel phosphodiesterase inhibitor, E6005 ointment, in healthy volunteers and in patients with atopic dermatitis. J Dermatol Treat. 2016;27(3):241–6.
Hanifin JM, Ellis CN, Frieden IG, Folster-Holst R, Stein Gold LF, Secci A, et al. OPA-15406, a novel, topical, nonsteroidal, selective phosphodiesterase-4 (PDE4) inhibitor, in the treatment of adult and adolescent patients with mild to moderate atopic dermatitis (AD): A phase-II randomized, double-blind, placebo-controlled study. J Am Acad Dermatol. 2016. doi:10.1016/j.jaad.2016.04.001.
Pettipher R, Hansel TT, Armer R. Antagonism of the prostaglandin D2 receptors DP1 and CRTH2 as an approach to treat allergic diseases. Nat Rev Drug Discov. 2007;6(4):313–25.
Bowton DL, Dmitrienko AA, Israel E, Zeiher BG, Sides GD. Impact of a soluble phospholipase A2 inhibitor on inhaled allergen challenge in subjects with asthma. J Asthma. 2005;42(1):65–71.
Leaker BR, Barnes PJ, O’Connor BJ, Ali FY, Tam P, Neville J, et al. The effects of the novel SHIP1 activator AQX-1125 on allergen-induced responses in mild-to-moderate asthma. Clin Exp Allergy. 2014;44(9):1146–53.
Slominski A, Pisarchik A, Zbytek B, Tobin DJ, Kauser S, Wortsman J. Functional activity of serotoninergic and melatoninergic systems expressed in the skin. J Cell Physiol. 2003;196(1):144–53.
Genentech. Genentech provides update on two identical phase III studies of Lebrikizumab in people with severe asthma [media release]. 2016 Feb 28 [online]. http://www.gene.com/media/press-releases/14619/2016-02-28/genentech-provides-update-on-two-identic. Accessed 15 Apr 2016.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Lisa A. Beck, MD, has acted as a consultant for Abbvie, Array BioPharma, Celgene, Genentech, Janssen, Medimmune, Novartis, Regeneron, Sanofi, Stiefel/GSK, and Unilever and owns Pfizer and Medtronic stock. She is Principal Investigator for clinical trials from Genentech and Regeneron and Co-Investigator for clinical trial from Xoma. Diane Wang, MD, is Co-Investigator for clinical trials from Genentech, Regeneron and Xoma.
Funding
Lisa A. Beck, MD received funding from NIH R21 grant (AR062357). Diane Wang, MD has no funding to declare.
Rights and permissions
About this article
Cite this article
Wang, D., Beck, L.A. Immunologic Targets in Atopic Dermatitis and Emerging Therapies: An Update. Am J Clin Dermatol 17, 425–443 (2016). https://doi.org/10.1007/s40257-016-0205-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-016-0205-5