Abstract
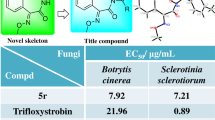
A series of novel thiazole Schiff base derivatives containing benzo[d][1,3]dioxole moiety was designed, synthesized and screened for their fungicidal activities. The preliminary results demonstrated that compounds 6p, 6q and 6r possessed potent activities against Phytophthora infestans, Pyricularia oryzae and Septoria tritici in vitro. Compounds 6d and 6r exhibited remarkable activities against Botrytis cinerea(whole plant) and Phytophthora infestans(leaf disk) respectively in vivo, which were identified as the most promising candidates for further study and could be used as possible lead compounds for developing new fungicides.
Similar content being viewed by others
References
Owen W. J., Sullenberger M. T., Loso M. R., Meyer K. G., Slanec T. J., Pest. Manag. Sci., 2015, 71, 83
Al-Ani I., Zimmermann S., Reichling J., Wink M., Phytomedicine, 2015, 22, 245
Wu C. C., Wu C. L., Huang S. L., Chang H. T., Wood Sci. Technol., 2011, 46, 737
Li X., Ma S., Eur. J. Med. Chem., 2015, 95, 1
Ayati A., Emami S., Asadipour A., Shafiee A., Foroumadi A., Eur. J. Med. Chem., 2015, 97, 699
Siddiqui N., Arshad M. F., Ahsan W., Alam M. S., Int. J. Pharm. Sci. Drug. Res., 2009, 1, 136
Peng J. M., Li W., Shen K., Huo S. F., Ye J., Hu A. X., Chem. J. Chinese Universities, 2013, 34(7), 1646
Luo X. F., Hu A. X., Wang Y., Ye J., Wang X., G., Ou X. M., Chem. J. Chinese Universities, 2011, 32(12), 2800
Altintop M. D., Ozdemir A., Turan-Zitouni G., Ilgin S., Atli O., Demirel R., Kaplancikli Z. A., Eur. J. Med. Chem., 2015, 92, 342
Amnerkar N. D., Bhongade B. A., Bhusari K. P., Arab. J. Chem., 2015, 8, 545
Farghaly T. A., Abdallah M. A., Masaret G. S., Muhammad Z. A., Eur. J. Med. Chem., 2015, 97, 320
Bharti S. K., Nath G., Tilak R., Singh S. K., Eur. J. Med. Chem., 2010, 45, 651
Aboul-Fadl T., Bin-Jubair F. A., Aboul-Wafa O., Eur. J. Med. Chem., 2010, 45, 4578
da Silva C. M., da Silva D. L., Modolo L. V., Alves R. B., de Resende M. A., Martins C. V. B., de Fátima Â., J. Adv. Res., 2011, 2, 1
Break M. K., Tahir M. I., Crouse K. A., Khoo T. J., Bioinorg. Chem. Appl., 2013, 2013, 1
Prakash C. R., Raja S., J. Saudi. Chem. Soc., 2013, 17, 337
Bohm H. J., Flohr A., Stahl M., Drug Discov. Today Technol., 2004, 1, 217
Vainio M. J., Kogej T., Raubacher F., Sadowsk J., J. Chem. Inf. Model., 2013, 53, 1825
Brown N., Mol. Inform., 2014, 33, 458
Jones G., Stanforth S. P., Organic Reactions, John Wiley & Sons, New Jersey, 1997, 1
Lin C. K., Lu T. J., Tetrahedron, 2010, 66, 9688
Wang Y., Hu A. X., Cao G., Li G. X., Zhang J. Y, Xia L., Ou X. M., Xu J., Chin. J. Org. Chem., 2008, 28, 443
Powell S., Wasserman W. J., J. Am. Chem. Soc., 1957, 79, 1934
Hu A. X., Qin Z., Chen P., Ye J., Chin. J. Org. Chem., 2010, 30, 923
Winter C., Rheinheimer J., Wolf A., Terteryan V., Poonoth M., Wiebe C., Kremzow-Graw D., Röhl F., Rohrer S. G., Wieja A., Strobilurin Type Compounds for Combating Phytopathogenic Fungi, WO2014202703 A1, 2014
Gewehr M., Stierl R., Niedenbruck M., Hunger U., Fungicidal Mixtures, US20080045414 A1, 2008
Ye J., Qiu S. Y., Hu A. X., Peng J. M., Qin Z., Chem. Res. Chinese Universities, 2014, 30(1), 49
Goswami S., Kaur R., Nagrale D. T., J. Appl. Nat. Sci., 2012, 4, 264
Binks P. R., Robson G. D., Goosey M. W., Trinci A. P. J., J. Gen. Microbiol., 1993, 139, 1371
Tvaruek L., Horáková P., Ji L., Acta Agrobot., 2005, 58, 79
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Key Technology R&D Program of China(No.2011BAE06B01).
Rights and permissions
About this article
Cite this article
Wu, Z., Ding, N., Lin, D. et al. Synthesis and fungicidal activity of some novel thiazole Schiff bases derived from benzo[d][1,3]dioxole. Chem. Res. Chin. Univ. 32, 49–54 (2016). https://doi.org/10.1007/s40242-016-5284-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-016-5284-6