Abstract
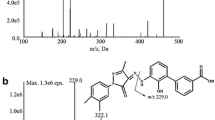
Astilbin is a potential immunosuppressive agent with minor cytotoxicity. Its oral bioavailability is supposed to be rather low and therefore a sensitive analytical method is required for its pharmacokinetic study after oral administration. A simple, sensitive and rapid liquid chromatography-tandem mass spectrometry(LC-MS/MS) method was developed and validated for the determination of astilbin in rat plasma. Plasma samples were subjected to liquid-liquid extraction with ethyl acetate and separated by reversed phase high performance liquid chromatography(HPLC) with methanol-0.01%(volume fraction) formic acid(50:50, volume ratio) as mobile phase. Quantitive determination was achieved on negative LC-MS/MS by a multiple reaction moitoring method with transitions m/z 449.1→150.9(quantifier) and m/z 449.1→284.9(qualifier) for astilbin and m/z 128.9→42.0 for internal standard(IS). A lower limit of quantification(LLOQ) of ng/mL was achieved within a short cycle time of 3.4 min. The method was successfully applied to a pharmacokinetic study involving oral and intravenous administrations of 6 mg/kg astilbin to six rats.
Similar content being viewed by others
References
Zhang Q. F., Cheung H. Y., Zeng L. B., J. Nat. Med., 2013, 67, 207
Zhang Q. F., Guo Y. X., Zheng G., Wang W. J., Nat. Prod. Res., 2013, 27, 277
Li X., Zhang Y. F., Yang L., Feng Y., Deng Y. H., Liu Y. M., Zeng X., Nat. Prod. Commun., 2012, 7, 181
Zheng Z. G., Duan T. T., He B., Tang D., Jia X. B., Wang R. S., Zhu J. X., Xu Y. H., Zhu Q., Feng L., J. Pharm. Biomed. Anal., 2013, 77, 44
Chen T., Li J., Cao J., Xu Q., Komatsu K., Namba T., Planta Med., 1999, 65, 56
Xu Q., Wu F., Cao J., Chen T., Jiang J., Saiki I., Koda A., Eur. J. Pharmacol., 1999, 377, 93
Cai Y., Chen T., Xu Q., J. Pharm. Pharmacol., 2003, 55, 691
Cai Y., Chen T., Xu Q., Inflamm. Res., 2003, 52, 334
Yi H. W., Lu X. M., Fang F., Wang J., Xu Q., Int. Immunopharmacol., 2008, 8, 1467
Closa D., Torres M., Hotter G., Bioque G., Leon O. S., Gelpi E., Rosello-Catafau J., Prostaglandins Leukot Essent Fatty Acids, 1997, 56, 331
Wang X. D., Meng M. X., Gao L. B., Liu T., Xu Q., Zeng S., Int. J. Pharm., 2009, 378, 1
Zhao R. Z., Zhao Y., Zhang L. Q., Lu C. J., Pak. J. Pharm. Sci., 2013, 26, 1
Yang X. H., Li H. B., Chen H., Li P., Ye B. P., Acta Pharm. Sinica, 2008, 43, 974
Chen L., Yin Y., Yi H., Xu Q., Chen T., J. Pharm. Biomed. Anal., 2007, 43, 1715
Tatsis E. C., Boeren S., Exarchou V., Troganis A. N., Vervoort J., Gerothanassis I. P., Phytochemistry, 2007, 68, 383
Guo J., Xu Q., Chen T., J. Chromatogr. B, 2004, 805, 357
Liang Y., Xu Q., Kang A., Xie Y., Xie T., Liu L., Hao H., Xie L., Wang G. J., J. Chromatogr. B, 2009, 877, 1765
US FDA, Bioanalytical Method Validation, Rockville, USA, http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm070107.pdf
Wang Y. W., Wang L., Gu J. K., Chen G., Zhong D. F., Zhou H., Chin. J. Anal. Chem., 2003, 31, 709
Zhang Q. F., Nie H. C., Shangguan X. C., Yin Z. P., Zheng G. D., Chen J. G., J. Agric. Food Chem., 2013, 61, 151
He Y., Liu H., Xie Z., Liao Q., Lai X., Du Z., Drug Dev. Ind. Pharm., 2013, doi: 10.3109/03639045.2012.756008
Mezghrani O., Ke X., Bourkaib N., Xu B. H., Pharmazie, 2011, 66, 754
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supported by the National Natural Science Foundation of China(No.81102383), the Science and Technology Major Specialized Projects for “Significant New Drugs Creation” of the 12th Five-year Plan of China(No.2012ZX09303-015) and the National Key Technology R&D Program of the Ministry of Science and Technology, China(No.2012BAI30B00).
Rights and permissions
About this article
Cite this article
Yin, L., Zhang, Yh., Zhao, S. et al. Rapid quantification of astilbin in rat plasma by liquid chromatography-tandem mass spectrometry and its application to pharmacokinetic study. Chem. Res. Chin. Univ. 29, 1078–1082 (2013). https://doi.org/10.1007/s40242-013-3166-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-013-3166-8