Abstract
Background
Various studies suggest that oxidative stress has a role in the etiology of diabetes mellitus (DM) and its complications. Detection of antioxidant enzymes and malondialdehyde (MDA) level in ocular fluid may provide the possible biomarkers for monitoring the progression of diabetic retinopathy (DR). The aim of this study was to compare catalase, glutathione peroxidase (GPx) and MDA levels in tears among diabetic patients with and without DR.
Methods
A cross-sectional study was conducted among type 2 DM patients. The patients were divided into three groups: no DR, non-proliferative DR (NPDR) and proliferative DR (PDR). Tears samples were collected using Schirmer strips for measurement of catalase, GPx and MDA.
Results
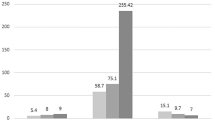
A total of 171 patients were recruited in this study (no DR, 58 patients; NPDR, 57 patients; PDR, 56 patients). There was significant difference in the mean level of GPx in tears between the three groups (no DR, 658.08 ± 115.70 U/L; NPDR, 653.78 ± 87.90 U/L; PDR, 605.31 ± 107.47 U/L, respectively) before and after adjustment for covariates (p = 0.013 and p = 0.001, respectively). Bonferroni post-hoc analysis showed PDR group had significantly lower mean GPx level than in no DR (p=0.001) and NPDR (p=0.037) after adjustment for covariates. There was no significant difference of mean catalase and MDA in the tears between the three groups before and after adjustment for covariates.
Conclusion
This study demonstrated that diabetic patient with DR is associated with low level of GPx in tears, suggesting that this antioxidant enzyme is a potential biomarker for predicting the presence of DR.
Similar content being viewed by others
Data availability
All the data and materials are contained within the manuscript.
Code availability
Not applicable.
References
Cai X, McGinnis JF. Diabetic retinopathy: animal models, therapies, and perspectives. J Diabetes Res. 2016;2016:3789217.
Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–64.
Yang QH, Zhang Y, Zhang XM, Li XR. Prevalence of diabetic retinopathy, proliferative diabetic retinopathy and non-proliferative diabetic retinopathy in Asian T2DM patients: a systemic review and Meta-analysis. Int J Ophthalmol. 2019;12(2):302–11.
Kim JH, Kwon HS, Park YM, Lee JH, Kim MS, Yoon KH, et al. Prevalence and associated factors of diabetic retinopathy in rural Korea: the Chungju Metabolic Disease Cohort Study. J Korean Med Sci. 2011;26(8):1068–73.
Rösen P, Nawroth PP, King G, Möller W, Tritschler HJ, Packer L. The role of oxidative stress in the onset and progression of diabetes and its complications: a summary of a Congress Series sponsored byUNESCO-MCBN, the American Diabetes Association and the German Diabetes Society. Diabetes Metab Res Rev. 2001;17(3):189–212.
Feldman EL. Oxidative stress and diabetic neuropathy: a new understanding of an old problem. J Clin Invest. 2003;111(4):431–3.
Jha JC, Banal C, Chow BSM, Cooper ME, Jandeleit-Dahm K. Diabetes and kidney disease: role of oxidative stress. Antioxid Redox Signal. 2016;25(12):657–84.
Calderon GD, Juarez OH, Hernandez GE, Punzo SM, De la Cruz ZD. Oxidative stress and diabetic retinopathy: development and treatment. Eye (Lond). 2017;31(8):1122–30.
Auten R, Davis J. Oxygen toxicity and reactive oxygen species: The devil is in the details. Pediatr Res. 2009;66:121–7. https://doi.org/10.1203/PDR.0b013e3181a9eafb.
Niedowicz DM, Daleke DL. The role of oxidative stress in diabetic complications. Cell Biochem Biophys. 2005;43(2):289–330. https://doi.org/10.1385/CBB:43:2:289.
Choy CK, Cho P, Chung WY, Benzie IF. Water-soluble antioxidants in human tears: effect of the collection method. Invest Ophthalmol Vis Sci. 2001;42(13):3130–4.
Haskins K, Bradley B, Powers K, Fadok V, Flores S, Ling X, et al. Oxidative stress in type 1 diabetes. Ann N Y Acad Sci. 2003;1005:43–54.
Karaouzene N, Merzouk H, Merzouk AS, Bouanane S, Loudjedi L, Mersouk SA. Interrelations between inflammatory and oxidative stress biomarkers in obese women with two complications (hypertension, diabetes). Rom J Diabetes Nutr Metab Dis. 2019;26(2):129–43.
Domènech EB, Marfany G. The relevance of oxidative stress in the pathogenesis and therapy of retinal dystrophies. Antioxidants (Basel). 2020;9(4):347.
Ezeiruaku FC, Wankasi MM, George GS. The antioxidant enzymes (superoxide dismutase; glutathione peroxidase; catalase) status in chronic and non-chronic diabetic mellitus type 1 and type 2 subjects in Yenegoa, Bayelsa State, Nigeria. J B Genet Res. 2016;2(1):34–44.
Briggs ON, Brown H, Elechi-amadi K, Ezeiruaku F, Nduka N. Superoxide dismutase and glutathione peroxidase levels in patients with long standing type 2 diabetes in Port Harcourt, Rivers State, Nigeria. Int J Sci Res (IJSR). 2016;5(3):1281–8.
Boussekine S, Menaceur F, Gasmi S, Lidoughi A, Rais T, Gattel H. Oxidative stress assessment and its relationship with the prevalence of atherogenic risk in patients with type 2 diabetes. J Diabetes Metab Disord. 2021;20(1):583–90. https://doi.org/10.1007/s40200-021-00785-4.
Kumawat M, Kharb S, Singh V, Singh N, Singh SK, Nada M. Plasma malondialdehyde (MDA) and antioxidant status in diabetic retinopathy. J Indian Med Assoc. 2014;112(1):29–32.
Njie-Mbye YF, Kulkarni-Chitnis M, Opere CA, Barrett A, Ohia SE. Lipid peroxidation: pathophysiological and pharmacological implications in the eye. Front Physiol. 2013;4:366. https://doi.org/10.3389/fphys.2013.00366.
Mancino R, Di Pierro D, Varesi C, Cerulli A, Feraco A, Cedrone C, et al. Lipid peroxidation and total antioxidant capacity in vitreous, aqueous humor, and blood samples from patients with diabetic retinopathy. Mol Vis. 2011;17:1298–304.
Brzović-Šarić V, Landeka I, Šarić B, Barberić M, Andrijašević L, Cerovski B, et al. Levels of selected oxidative stress markers in the vitreous and serum of diabetic retinopathy patients. Mol Vis. 2015;21:649–64.
Koh YN, Zunaina E, Liza-Sharmini AT, Abd-Aziz CB, Che-Maraina CH, Chong MF, et al. Antioxidant enzymes in tears among Malay age-related macular degeneration patients. Mal J Med Health Sci. 2020;16(2):149–56.
Azhan A, Zunaina E, Mahaneem M, Siti-Azrin AH. Comparison of VEGF level in tears post phacoemulsification between non-proliferative diabetic retinopathy and non-diabetic patients. J Diabetes Metab Disord. 2021. https://doi.org/10.1007/s40200-021-00875-3.
Ang WJ, Zunaina E, Norfadzillah AJ, Raja-Norliza RO, Julieana M, Ab-Hamid SA, et al. Evalution of vascular endothelial growth factor levels in tears and serum among diabetic patients. PLoS One. 2019;14(8):e0221481.
Horwath-Winter J, Kirchengast S, Meinitzer A, Wachswender C, Faschinger C, Schmut O. Determination of uric acid concentrations in human tear fluid, aqueous humour and serum. Acta Ophthalmol. 2009;87:188–92. https://doi.org/10.1111/j.1755-3768.2008.01215.x.
Wilkinson CP, Ferris FL 3rd, Klein RE, Lee PL, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677–82.
Beyazyıldız E, Cankaya AB, Ergan E, Anayol MA, Ozdamar Y, Sezer S, et al. Changes of total antioxidant capacity and total oxidant status of aqueous humor in diabetes patients and correlations with diabetic retinopathy. Int J Ophthalmol. 2013;6(4):531–6.
Hartnett ME, Stratton RD, Browne RW, Rosner BA, Lanham RJ, Armstrong D. Serum markers of oxidative stress and severity of diabetic retinopathy. Diabetes Care. 2000;23(2):234–40.
Said NS, Hadhoud KM, Nada WM, El Tarhouny SA. Superoxide dismutase, glutathione peroxidase and vitamin E in patients with diabetic retinopathy. Life Sci J. 2013;10(1):1851–6.
Sharma S, Saxena S, Srivastav K, Shukla RK, Mishra N, Meyer CH, et al. Nitric oxide and oxidative stress is associated with severity of diabetic retinopathy and retinal structural alterations. Clin Exp Ophthalmol. 2015;43(5):429–36.
Kesavulu MM, Giri R, Rao BK, Apparao C. Lipid peroxidation and antioxidant enzyme levels in type 2 diabetics with microvascular complications. Diabetes Metab. 2000;26(5):387–92.
Darmaun D, Smith SD, Sweeten S, Sager BK, Welch S, Mauras N. Evidence for accelerated rates of glutathione utilization and glutathione depletion in adolescents with poorly controlled type 1 diabetes. Diabetes. 2005;54(1):190–6.
Robaczewska J, Kedziora-Kornatowska K, Kozakiewicz M, Zary-Sikorska E, Pawluk H, Pawliszak W, et al. Role of glutathione metabolism and glutathione-related antioxidant defense systems in hypertension. J Physiol Pharmacol. 2016;67(3):331–7.
de Haan JB, Stefanovic N, Nikolic-Paterson D, Scurr LL, Croft KD, Mori TA, et al. Kidney expression of glutathione peroxidase-1 is not protective against streptozotocin-induced diabetic nephropathy. Am J Physiol Renal Physiol. 2005;289(3):F544–51. https://doi.org/10.1152/ajprenal.00088.2005.
Pavlovschi E, Pantea V, Borovic D, Tagadiuc O. Tear and serum superoxide dismutase and catalase activities in hypertensive retinopathy. Russ Open Med J. 2021;10:e0305.
Cojocaru IM, Cojocaru M, Muşuroi C, Botezat M, Lazăr L, Drută A. Lipid peroxidation and catalase in diabetes mellitus with and without ischemic stroke. Rom J Intern Med. 2004;42(2):423–9.
Góth L. Catalase deficiency and type 2 diabetes. Diabetes Care. 2008;31(12):e93.
Sözmen EY, Sözmen B, Delen Y, Onat T. Catalase/superoxide dismutase (SOD) and catalase/paraoxonase (PON) ratios may implicate poor glycemic control. Arch Med Res. 2001;32(4):283–7.
Maleki S, Falsafi P, Pakdel F, Eslami H, Ahari UZ, Pouralibaba F, et al. A comparison between catalase and salivary alpha-amylase level in patients with type I diabetes and non-diabetic people. Biomed Pharmacol J. 2016;9(2):463–8.
Aouacheri O, Saka S, Krim M, Messaadia A, Maidi I. The investigations of the oxidative stress-related parameters in type 2 diabetes mellitus. Can J Diabetes. 2015;39(1):44–9.
Mahmoud Yousif SM, Abdalla MS, Elmahdi EM. Oxidant/antioxidant status of Sudanese type II diabetic patients with multiple complications. J Diabetol. 2019;10:69–75.
Pan HZ, Zhang H, Chang D, Li H, Sui H. The change of oxidative stress products in diabetes mellitus and diabetic retinopathy. Br J Ophthalmol. 2008;92(4):548–51.
Rani K, Gavel P, Bharti S. MDA, oxidative stress marker-role in diabetic nephropathy with special reference to type II diabetes mellitus. Indian J Appl Res. 2016;6(5):128–30.
Slatter DA, Bolton CH, Bailey AJ. The importance of lipid-derived malondialdehyde in diabetes mellitus. Diabetologia. 2000;43(5):550–7 (0200-021-00785-4).
Acknowledgements
The authors thank staff from Immunology Lab, School of Medical Sciences, Universiti Sains Malaysia, for their technical assistance for this study. A special thanks to Dr Wan Nor Ariffin Wan Mansor, Biostatistics and Research Methodology Unit, Universiti Sains Malaysia, for his assistance and advice of the statistical analysis.
Funding
This research was partially supported by Research University Grant from Universiti Sains Malaysia (grant no: 1001/PPSP/812194).
Author information
Authors and Affiliations
Contributions
KKH: Conceptualization, data curation, formal analysis, methodology, writing—original draft. EZ: Conceptualization, data curation, formal analysis, funding acquisition, methodology, writing—review & editing, supervision, project administration. HH: Conceptualization, formal analysis, methodology, supervision. AACB: Conceptualization, data curation, formal analysis, methodology, supervision. CHCM: Conceptualization, data curation, formal analysis, methodology, supervision.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Institutional Review Board (IRB) of Universiti Sains Malaysia (USM) [ref: USM/ Jawatankuasa Etika Penyelidikan Manusia (JEPeM)/276.2. (4)] and followed the tenets of the declaration of Helsinki. Written and informed consent of participants was obtained for each patient prior to the study.
Consent to participate
Not applicable.
Consent for publication
All authors approved the manuscript for publication.
Competing interests
The authors declare that they have no competing interests.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kwong-Han, K., Zunaina, E., Hanizasurana, H. et al. Comparison of catalase, glutathione peroxidase and malondialdehyde levels in tears among diabetic patients with and without diabetic retinopathy. J Diabetes Metab Disord 21, 681–688 (2022). https://doi.org/10.1007/s40200-022-01030-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-022-01030-2