Abstract
Background
Among the different types of cancers, breast cancer, bone cancer and cervical cancer are the most common gender specific cancer types that are affecting the women worldwide. Currently, many enzymatic and cellular pathways are known as drug targets for the treatment of cancer. Even though many improvements have been made in the therapy of various types of cancer, but the major disadvantage of available anti-cancer drugs is their non-selective behavior towards cancer cells as well as normal cells.
Objectives
In the light of this fact, the searching of new compounds with selective behavior only towards cancer cells is critically important. Previously, we have identified several series of compounds as the potential inhibitors of these families.
Methods
Herein, we investigate quinolones and quinolines for their anti-cancer activity against breast cancer cells (MCF–7), bone marrow cancer cells (K–562) and cervical cancer cells (HeLa) by MTT assay. The most effective derivatives were further subjected to flow cytometry analysis followed by fluorescence microscopic analysis by using 4´,6-diamidine-2´-phenylindole (DAPI) and propidium staining (PI) staining.
Results
All the tested compounds were found selective only towards cancer cells. The identified compounds also induced either G2 or S-phase cell cycle arrest within the respective cancer cell line, chromatin condensation and the nuclear fragmentation, as well as maximum interaction with DNA.
Conclusions
These results provide evidence that the characteristic chemical features of attached groups are the key factors for their anticancer effects and play a useful role in revealing the mechanisms of action in relation to the known compounds in future research programs.

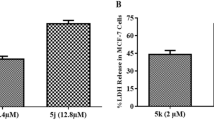
Flow cytometric analysis of cell cycle using propidium iodide staining. Cell apoptosis observed under fluorescence microscope using DAPI and PI staining.









Similar content being viewed by others
References
Nepali K, Sharma S, Sharma M, Bedi PMS, Dhar KL. 2014. Rational approaches, design strategies, structure activity relationship and mechanistic insights for anticancer hybrids. Eur J Med Chem. 2014;77(4):422–87.
Fortin S, Bérubé G. Advances in the development of hybrid anticancer drugs. Expert Opinion Drug Discov. 2013;8(2):1029–47.
Raj T, Bhatia RK, Sharma M, Saxena AK, Ishar MPS. Cytotoxic activity of 3-(5-phenyl-3H-[1, 2, 4] dithiazol-3-yl) chromen-4-ones and 4-oxo-4H-chromene-3-carbothioic acid N-phenylamides. Eur J Med Chem. 2010;45(4):790–4.
Danaei G, Vander Hoorn S, Lopez AD, Murray CJ, Ezzati M. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366(2):1784–93.
Kateb B, Chiu K, Black KL, Yamamoto V, Khalsa B, Ljubimova JY, et al. Nanoplatforms for constructing new approaches to cancer treatment, imaging, and drug delivery: what should be the policy? Neuroimage. 2011;54(2):S106–24.
Nepali K, Sharma S, Kumar D, Budhiraja A, Dhar KL. Anticancer hybrids-a patent survey. Recent Pat Anti-cancer Drug Discov. 2014;9(3):303–39.
Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedeberg's Arch Pharmacol. 2000;362(4–5):299–309.
Vitaku E, Smith DT, Njardarson JT. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among US FDA approved pharmaceuticals: miniperspective. J Med Chem. 2014;57(24):10257–74.
Sondhi SM, Johar M, Rajvanshi S, Dastidar SG, Shukla R, Raghubir R, et al. Anticancer, anti-inflammatory and analgesic activity evaluation of heterocyclic compounds synthesized by the reaction of 4-isothiocyanato-4-methylpentan-2-one with substituted o-phenylenediamines, o-diaminopyridine and (un) substituted o. Aust J Chem. 2001;54(1):69–74.
Zimmermann H, Zebisch M, Sträter N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012;8(3):437–502.
Yegutkin GG. Enzymes involved in metabolism of extracellular nucleotides and nucleosides: functional implications and measurement of activities. Crit Rev Biochem Mol Biol. 2014;49(6):473–97.
Ausekle E, Ejaz SA, Khan SU, Ehlers P, Villinger A, Lecka J, et al. New one-pot synthesis of N-fused isoquinoline derivatives by palladium-catalyzed C–H arylation: potent inhibitors of nucleotide pyrophosphatase-1 and-3. Org Biomol Chem. 2016;14(48):11402–14.
Khan I, Shah SJ, Ejaz SA, Ibrar A, Hameed S, Lecka J, et al. Investigation of quinoline-4-carboxylic acid as a highly potent scaffold for the development of alkaline phosphatase inhibitors: synthesis, SAR analysis and molecular modelling studies. RSC Adv. 2015;5(79):64404–13.
Miliutina M, Ejaz SA, Khan SU, Iaroshenko VO, Villinger A, Iqbal J, et al. Synthesis, alkaline phosphatase inhibition studies and molecular docking of novel derivatives of 4-quinolones. Eur J Med Chem. 2017;126:408–20.
Orimo H. The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J Nippon Med Sch. 2010;77(1):4–12.
Wanichpakorn S, Kedjarune-Laggat U. Primary cell culture from human oral tissue: gingival keratinocytes, gingival fibroblasts and periodontal ligament fibroblasts. SJST. 2010;32(4):327–31.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1–2):55–63.
Niks M. Towards an optimized MTT assay. J Immunol Methods. 1990;130(2):149–51.
Hassan S, Ejaz SA, Saeed A, Shehzad M, Khan SU, Lecka J, et al. 4-Aminopyridine based amide derivatives as dual inhibitors of tissue non-specific alkaline phosphatase and ecto-5′-nucleotidase with potential anticancer activity. Bioorg Chem. 2018;76(5):237–48.
Saito Y, Uchida N, Tanaka S, Suzuki N, Tomizawa-Murasawa M, Sone A, et al. Induction of cell cycle entry eliminates human leukemia stem cells in a mouse model of AML. Nature biotechnol. 2010;28(3):275–80.
Iqbal J, Ejaz SA, Saeed A, al Rashida M. Detailed investigation of anticancer activity of sulfamoyl benz (sulfon) amides and 1H–pyrazol–4–yl benzamides: An experimental and computational study. Eur J Pharmacol. 2018;832:11–24.
Lin GJ, Jiang GB, Xie YY, Huang HL, Liang ZH, Liu YJ. Cytotoxicity, apoptosis, cell cycle arrest, reactive oxygen species, mitochondrial membrane potential, and Western blotting analysis of ruthenium (II) complexes. J Biol Inorg Chem. 2013;18(8):873–82.
Sirajuddin M, Ali S, McKee V, Zaib S, Iqbal J. Organotin (IV) carboxylate derivatives as a new addition to anticancer and antileishmanial agents: design, physicochemical characterization and interaction with Salmon sperm DNA. RSC Adv. 2014;4(2):57505–21.
MOE, version 2014.0901, Chemical Computing Group (CCG), Montreal, Canada, http://www.chemcomp.com/MOEMolecular_Operating_Environment.html. Accessed August 2016.
Visualizer, D.S. 2005. Accelrys Software Inc, Discovery Studio Visualizer, 2.
Acknowledgements
J.I. is thankful to the Higher Education Commission of Pakistan for the financial support through Project No.Ph-V-MG-3/Peridot/R&D/HEC/2019 and 6927/NRPU/R&D/17.
Author information
Authors and Affiliations
Contributions
Syeda Abida Ejaz performed the biochemical assays and drafted the manuscript under the supervision of Jamshed Iqbal. Imtiaz Khan, Elina Ausekle, Mariia Miliutina and Peter Langer provided the compounds for the study. All the authors checked and finally approved the draft before submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors confirm that this article content has no conflict of interest. The authors also declare no competing financial interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Iqbal, J., Ejaz, S.A., Khan, I. et al. Exploration of quinolone and quinoline derivatives as potential anticancer agents. DARU J Pharm Sci 27, 613–626 (2019). https://doi.org/10.1007/s40199-019-00290-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40199-019-00290-3