Abstract
Kidney disease affects millions of people and represents a growing health care burden. There is no cure for loss of kidney function, because humans are born with a finite number of nephrons with limited ability to repair themselves after injury. Zebrafish kidneys have the remarkable ability to regenerate after injury by adding new nephrons as well as repairing existing nephrons as mammals do. In fish, new nephrons are able to differentiate in situ and functionally connect to the existing tubular architecture throughout the life of the adult animal. Zebrafish kidney regeneration allows investigation of common mechanisms for repair of nephrons after injury while giving insights into completely unique mechanisms for reserving and maintaining a multipotent progenitor cell population. In this review, we give an overview of zebrafish kidney development and regeneration, explore available genetic tools, survey different injury models, and consider implications for human kidney regeneration.
Similar content being viewed by others
Introduction
The zebrafish is well known for its exceptional regenerative powers. Its small size and amenability to genetic manipulation make it ideal for the study of regeneration in a vertebrate adult organism. Especially fascinating is its capacity to regenerate organs such as heart and appendages, which defeat the abilities of mammals. Zebrafish also have the ability to regenerate their kidneys, and several recent advances in genetic tools and molecular markers promise to expand the utility of zebrafish as a model to study kidney regeneration.
The human kidney maintains fluid homeostasis, regulates blood pressure and filters waste from the body. The functional unit of the kidney is the nephron, and humans are born with a finite number of nephrons (800,000–1,200,000), which for most people is enough for a healthy lifetime [1]. However, premature birth or developmental defects resulting in renal hypodysplasia or agenesis, acute kidney injury due to infection or toxins, chronic kidney injury resulting in end-stage renal disease due to hypertension or diabetes, and renal cancers all result in loss of kidney function. Humans do not produce new nephrons and have only a limited ability to repair existing tubules after injury. Currently, the only treatment options for renal insufficiency are renal replacement therapy (dialysis) or kidney transplant. Kidney disease is a growing burden on the health care system in the United States with almost 1 million people living with end-stage renal disease, and although 18,000 kidney transplants are performed each year, the wait list is 100,000 people and growing [2, 3]. With more premature infants surviving to become adults, more people living with chronic diseases, and longer lifespans, more people are outliving the usefulness of their kidneys. Therapies that can enhance nephron repair or enable the generation of new nephrons are required to meet their needs.
Kidney Development and Regeneration in Zebrafish
Zebrafish retain the ability to produce new nephrons throughout adult life. In vertebrates, the pronephros is the first kidney that forms. It is the functional kidney of the developing fish and consists of a single pair of nephrons that share a central glomerulus [4]. Subsequently, posterior to the pronephros the mesonephros forms. Experiments in chick embryos show that the nephric duct induces the nearby mesenchyme to undergo mesenchymal to epithelial transition (MET) to produce mesonephric tubules, and recently it has been shown that the tubule induction relies on Wnt signaling [5–7]. In fish, this mesonephros is the final adult kidney (Fig. 1a–c). Mammals, which produce a highly branched metanephros for use in postnatal life, retain the mesonephros as a transient embryonic structure that later contributes to the adult reproductive system. Although mammals are able to repair existing metanephric nephrons after injury through proliferation of differentiated tubular epithelium [8–11], they are unable to form new nephrons, and as yet nothing fulfilling the requirements of a kidney stem cell has been found [12]. In contrast to mammals, adult fish are able to undergo neonephrogenesis and form new nephrons in response to injury [13–18].
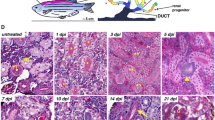
a In the zebrafish adult mesonephric kidney, the anterior head kidney is the primary site of blood formation, while the bulk of nephrons is present in the trunk region and drains into the collecting ducts, which then empty into the cloaca. b Whole-mount in situ hybridization for slc4a2a reveals nephron epithelium and collecting ducts, while nephrin is expressed in podocytes in glomeruli. c Higher magnification of box in B shows the loose, irregular organization of tubules. d Black and white photograph of a normal wild-type TuAB adult female (above) compared to adult female with edema resulting from severe kidney failure (below) 5 days after gentamicin-induced acute kidney injury. Note the scales raised perpendicular to the skin because of swelling all over the body (arrowheads). Later dissection revealed obstruction of both collecting ducts, presumably from sloughed tissue. e–i Methylene blue/azure II staining of polymer embedded tissue sections from gentamicin injured zebrafish kidneys. e After gentamicin injury, tubules are filled with casts (asterisk) of sloughed epithelial tissue, and f glomeruli show evidence of scarring and fibrosis (arrowheads). g Darkly staining, basophilic aggregate in close proximity to an existing tubule (arrowhead). h Elongating immature nephron (arrowhead). i Immature nephron after undergoing convolution (arrowheads)
After insult with the nephrotoxin gentamicin there is often no visible outward manifestation of injury because the fish is able to repair and regenerate its kidneys. In cases of severe injury, adult zebrafish can exhibit edema (Fig. 1d). This edema often resolves quickly, and the fish goes about its normal activities. This is remarkable, given the effects of gentamicin, which include necrosis and sloughing of tubular epithelium and scarring of glomeruli (Fig. 1e, f). Injury is rapidly followed by proliferation and repair of existing tubules and a wave of new nephron formation. Histologically, the first evidence of new nephron formation is a strongly basophilic cluster of cells adjacent to an existing tubule (Fig. 1g). This aggregate undergoes proliferation to form a structure similar to a renal vesicle, which then elongates, convolutes and differentiates into an immature nephron (Fig. 1h, i). This new nephron plumbs into the existing distal tubule structure at the base and recruits blood vessels to form a new glomerulus at the tip [13, 19•]. This process is in some respects similar to the formation of the renal vesicle from the condensed metanephric mesenchyme and invasion of the branched ureter by the distal tip of the S-shaped body [20]. It may be that in the regenerating zebrafish kidney, injury signals from injured tubules stimulate and recruit progenitor cells to form renal condensates, analogous to the role of the Wnt9b-expressing ureteric epithelium in the metanephros [21]. However, currently little is known about injury signals that stimulate neonephrogenesis. Evidence for a systemic proliferative signal has been suggested in the skate kidney injury model where unilateral kidney resection induced contralateral kidney growth [16, 22]. Another candidate source for regenerative signals may be inflammatory cells recruited by injury [23, 24]. Although further studies will be required to resolve these possibilities, the robust and reproducible regenerative response to renal injury in zebrafish makes it an attractive system to identify factors that stimulate nephron regeneration.
Imaging, Transgenic Lines and Genome Editing
Some of the well-known strengths of zebrafish as a model system are the transparency of the embryos and the ability to do forward mutagenesis screens and high-throughput chemical screens. There are many existing fluorescent lines available for live/time lapse imaging in the zebrafish kidney [25, 26]. These transgenic lines express fluorescent proteins in discrete cell types and regions of the nephron. Although these have been primarily used to study pronephric kidney development, they could be adapted to study mesonephric development as well as regeneration. Of particular interest are transgenic lines incorporating the wt1b promoter and lhx1a promoter, which label progenitor cells [18, 19•, 27, 28]. Additionally, casper mutant fish, which are lacking melanocytes and iridophores, are largely transparent and may be useful for live imaging in deeper tissues such as the kidney [29].
Forward genetic screens have produced a number of mutants relating to kidney function [30, 31]. These mutants were characterized based on the display of cystic phenotypes and have led to the identification of multiple genes related to cilia assembly and function. However, since the zebrafish larva relies on a pronephros consisting of a single pair of nephrons and the first mesonephric nephrons are not formed until day 12–14 post fertilization [18], mutations that affect nephric specification and differentiation would lead to loss of kidney function, non-cystic edema and early death in the larva [32]. Mutations specifically affecting adult kidney regeneration have yet to be discovered. A chemical screen performed on zebrafish embryos using lhx1a expression in the intermediate mesoderm as an endpoint resulted in the discovery that HDAC inhibitors expand renal progenitor cell populations [33]. These same compounds were subsequently shown to enhance renal function in mammalian injury models [34, 35]. Automated systems have been developed for high throughput imaging of embryonic or larval kidneys expressing reporter transgenes [36–38]. Combined with chemical screening, such high throughput systems have the potential to identify additional candidate small molecules, which could then be validated for effects on adult fish kidneys.
Where the fish excels is ease of generating transgenic animals. The Tol2 Gateway system makes production of new transgenic lines relatively simple in zebrafish [39–41]. Recent advances in zebrafish trangenesis have focused on tamoxifen-inducible Cre recombinase (CreERT) transgenes as well as better promoters for ubiquitous expression of transgenes [42]. Now a tissue-specific promoter can be used to drive CreERT, and in conjunction with a ubiquitously expressed transgene containing a stop sequence flanked by loxP sites, tamoxifen induction allows Cre-mediated excision of the stop sequence and gene expression. Not only reporter genes but also effector genes for cell ablation or constitutively active/dominant negative forms of genes can also be expressed. For example, a recent paper studying heart regeneration combines a tissue-specific promoter with a bioluminescent reporter (Zebraflash) as well as CreERT-mediated expression of diptheria toxin A for cell ablation [43].
CreERT transgenes also allow lineage-tracing experiments to be performed. Various switching lines exist, where Cre-mediated excision leads to permanent switching of reporter colors in cells and populations of cells can be followed to determine fate. Zebrabow transgenes have been created in the emulation of Brainbow in mice, allowing clonal analysis of progenitor cell fates [44–46]. Promoters driving expression in putative progenitor cells can now be used to perform definitive lineage tracing studies investigating their contribution to new nephron formation.
Until recently, it has not been possible to create genetic knockouts/knockins in zebrafish as in mice because of the lack of ES cell lines for performing homologous recombination. The recent development of genome editing tools such as the transcription activator-like effector nucleases (TALENs) and CRISPR/Cas9 systems has rapidly opened up new opportunities for targeted mutation in zebrafish. TALENs have been highly effective in zebrafish for creating targeted mutations in specific genes [47–50]. A newer method of creating targeted mutations caused by double-strand break repair using the Crispr/Cas9 system is also highly effective in zebrafish [51–53]. The small insertions and deletions are created at high efficiency, and the design of guide RNAs is much easier than TALEN assembly. Insertion of small sequences such as loxP sites based on homologous recombination to the target sequence is also possible [48]. In combination with CreERT transgenes, this approach opens up the possibility of making conditional knockouts in zebrafish, allowing the study of loss of genetic function in adult tissues.
Injury Models
Surgical injury models are commonly used to induce kidney injury in mice such as unilateral ureteric obstruction and kidney ischemia reperfusion [54, 55]. As noted above, surgical nephrectomy in skates has been shown to induce kidney hypertrophy as well as new nephron production in the remaining kidney tissue [16, 22]. Nephrectomy in an adult zebrafish is difficult because of its small size and the close proximity of the aorta. Nonetheless, ureteric obstruction has been performed in the adult zebrafish using crush injury to the distal collecting ducts just anterior to the cloaca [56]. In this study, subsequent distension and stretch of kidney nephrons were linked to induction of the ciliogenic transcription factor foxj1a, suggesting a potential role for ciliogenesis in stretch injury responses.
The aminoglycoside gentamicin is a widely used antibiotic with known nephrotoxic and ototoxic effects in humans [57]. Intraperitoneal injection of gentamicin is an established method of inducing acute kidney injury in fish [13]. This injury in fish mimics the loss of tubular epithelium and scarring of glomeruli that occur in humans after gentamicin overdose [58]. After acute injury of the zebrafish kidney with gentamicin, there is a synchronous wave of new nephron formation, such that specific timepoints after injury allow investigation of specific stages of new nephron formation. New nephrons are continuously formed at a very low rate in the adult, and 0–2 pre-tubular aggregates can be expected normally in an uninjured fish; however, this can be easily distinguished from an injury response where 50–150 aggregates indicate widespread neonephrogenesis [18]. New nephron formation in the context of an adult kidney allows investigation of signals required for proliferation, differentiation and elongation of new nephrons, as well as maintenance of a self-renewing nephron progenitor population.
A seminal paper by Diep et al. [19•] used FACS and cell transplantation to examine the progenitor potential of cells in the kidney. The authors found that undifferentiated nephron progenitors (cadherin17−, lhx1a+) could be transplanted into injured hosts and form new nephrons. However, although the lhx1a:gfp transgene is expressed in single cells as well as aggregates, the minimum number of transplanted cells that could form a new nephron was 10–30 cells, indicating that although lhx1a+ aggregates were able to form new nephrons, single lhx1a+ cells were not able to do this. Mixing GFP+ and mCherry+ cells together before transplantation resulted in new nephrons that were either a single color or a mosaic of both colors, indicating that more than one cell could contribute to a new nephron, but not ruling out the possibility that a single cell could give rise to a whole nephron. It is unclear what the functional difference is between the lhx1a+ single cells and lhx1a+ cells in aggregates. It is possible that progenitor cell aggregates benefit from a “niche” effect supplied by adjacent tubules or from a “community” effect (autocrine factors) from other progenitor cells in the cluster. It also remains possible that only a subset of cells in a renal progenitor aggregate perform as true stem cells, while the other lhx1a+ cells represent a more differentiated, transit amplifying population.
A particularly effective method for studying the function of a particular cell type in zebrafish is by conditional targeted cell ablation using the nitroreductase/metronidazole system [59]. A promoter of choice is used to drive expression of the bacterial enzyme nitroreductase, usually fused to a fluorescent reporter for easy visualization. Administration of the prodrug metronidazole in the fish water results in nitroreductase-mediated production of reactive oxygen species, resulting in cell death. Washing out the metronidazole allows recovery of the tissue. This system has been used effectively to ablate podocytes in zebrafish using a podocin promoter [60, 61•]. Ablation of podocytes resulted in edema and proteinuria as well as podocyte foot process effacement. Podocyte ablation was performed in the adult fish with similar results [61•]. After injury in the adult, wt1b expression was reinitiated in cells of Bowman’s capsule, suggesting a revival of developmental pathways in repair of the glomerulus. These authors also developed a novel assay for integrity of the glomerular filtration barrier, in which a vitamin D-binding protein fused to GFP and normally expressed in serum was excreted and detected by ELISA after injury, indicating proteinuria. This is an alternative to injection of fluorescently labeled dextran into the circulation of the fish [58, 62]. This paper demonstrates how both injury induction and a functional assay for injury can be encoded genetically using transgenes, replacing the need for injections of toxins or fluorescent dextrans and minimizing handling of the fish.
Implications for Human Kidney Regeneration
Human iPS cells offer the promise of taking a patient’s own cells and differentiating them into kidney tissue to restore kidney function. However, integrating different cell types into the structure of a nephron and directing that nephron to functionally connect with the existing vasculature and collecting duct system will rely on proper intrinsic cues from the cells or external cues from their environment. Rapid strides have been made in differentiating iPS cells into various nephric cell types based on knowledge of kidney development. This approach uses chemicals and secreted growth factors to differentiate cells in a stepwise process through mesendoderm and intermediate mesoderm into ureteric bud-like or metanephric mesenchyme-like cells [63–66]. Similar approaches might be used to progress further into more differentiated cell types, especially the more elusive proximal tubule cells [67] or even podocytes [68]. One option for providing external cues uses decellularized organs as scaffolds and repopulating them with epithelial and endothelial cells to make an implantable, bioengineered kidney [69]. Until the essential matrix components can be reproduced independently, finding sources of organs to use as scaffolds may be difficult. Another hurdle yet to be addressed by iPS approaches is how new nephrons will be integrated with the pre-existing collecting duct system to permit functional fluid filtration and urine production. The adult zebrafish fish kidney, as an in vivo model of new nephron formation in the context of an existing kidney structure, offers the potential to decode the signals required to direct nephron differentiation and functional integration.
Conclusions
Zebrafish kidney regeneration gives us an opportunity to study new nephron formation in an adult vertebrate. An expanded repertoire of new transgenic and genome editing tools is giving us a better molecular understanding of kidney regeneration in the fish and points the way to insights that can shape efforts to reproduce or engineer kidney regeneration in humans.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Hallgrimson B, Hallgrimur B, Vize PD (2003) In: Vize PD, Woolf AS, Bard JBL (eds) The kidney: from normal development to congenital disease. Academic Press, New York, pp 149–164
Prevention CFD (2010) National chronic kidney disease fact sheet: general information and national estimates on chronic kidney disease in the United States, http://www.cdc.gov/DIABETES//pubs/factsheets/kidney.htm (2010)
Services UD (2013) National transplantation data report. OPTN: Data Organ Procurement and Transplantation Network, http://optn.transplant.hrsa.gov/latestData/step2.asp
Drummond IA (2000) The zebrafish pronephros: a genetic system for studies of kidney development. Pediatr Nephrol 14:428–435
Waddington CH (1938) The morphogenetic function of a vestigial organ in the chick. J Exp Biol 15:255–260
Grünwald P (1937) Zur entwicklungsmechanick der Urogenital-system beim Huhn. Wilhelm Roux Archiv fur Entwicklungsmechanik der Organismen 136:786–813
Soueid-Baumgarten S, Yelin R, Davila EK, Schultheiss TM (2014) Parallel waves of inductive signaling and mesenchyme maturation regulate differentiation of the chick mesonephros. Dev Biol 385:122–135. doi:10.1016/j.ydbio.2013.09.026
Humphreys BD et al (2008) Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2:284–291
Humphreys BD et al (2011) Repair of injured proximal tubule does not involve specialized progenitors. Proc Natl Acad Sci USA 108:9226–9231. doi:10.1073/pnas.1100629108
Guo JK, Cantley LG (2010) Cellular maintenance and repair of the kidney. Annu Rev Physiol 72:357–376. doi:10.1146/annurev.physiol.010908.163245
Cirio MC, de Groh ED, de Caestecker MP, Davidson AJ, Hukriede NA (2013) Kidney regeneration: common themes from the embryo to the adult. Pediatr Nephrol. doi:10.1007/s00467-013-2597-2
Little MH, Bertram JF (2009) Is there such a thing as a renal stem cell? J Am Soc Nephrol 20:2112–2117. doi:10.1681/ASN.2009010066
Reimschuessel R (2001) A fish model of renal regeneration and development. ILAR J 42:285–291
Reimschuessel R, Bennett RO, May EB, Lipsky MM (1990) Development of newly formed nephrons in the goldfish kidney following hexachlorobutadiene-induced nephrotoxicity. Toxicol Pathol 18:32–38
Reimschuessel R, Williams D (1995) Development of new nephrons in adult kidneys following gentamicin-induced nephrotoxicity. Ren Fail 17:101–106
Elger M et al (2003) Nephrogenesis is induced by partial nephrectomy in the elasmobranch Leucoraja erinacea. J Am Soc Nephrol 14:1506–1518
Watanabe N et al (2009) Kidney regeneration through nephron neogenesis in medaka. Dev Growth Differ 51:135–143. doi:10.1111/j.1440-169X.2009.01090.x
Zhou W, Boucher RC, Bollig F, Englert C, Hildebrandt F (2010) Characterization of mesonephric development and regeneration using transgenic zebrafish. Am J Physiol Renal Physiol 299:F1040–F1047. doi:10.1152/ajprenal.00394.2010
• Diep CQ, et al. (2011) Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Nature 470:95–100. doi:10.1038/nature09669. This paper is the first to rigorously identify progenitor cells capable of self-renewal and production of all nephron cell types in the adult zebrafish kidney
Kao RM, Vasilyev A, Miyawaki A, Drummond IA, McMahon AP (2012) Invasion of distal nephron precursors associates with tubular interconnection during nephrogenesis. J Am Soc Nephrol 23:1682–1690. doi:10.1681/ASN.2012030283
Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP (2005) Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell 9:283–292. doi:10.1016/j.devcel.2005.05.016
Drummond I (2003) The skate weighs in on kidney regeneration. J Am Soc Nephrol 14:1704–1705
Lieschke GJ, Oates AC, Crowhurst MO, Ward AC, Layton JE (2001) Morphologic and functional characterization of granulocytes and macrophages in embryonic and adult zebrafish. Blood 98:3087–3096
Renshaw SA et al (2006) A transgenic zebrafish model of neutrophilic inflammation. Blood 108:3976–3978. doi:10.1182/blood-2006-05-024075
Vasilyev A et al (2009) Collective cell migration drives morphogenesis of the kidney nephron. PLoS Biol 7:e9. doi:10.1371/journal.pbio.1000009
Vasilyev A, Drummond IA (2012) Live imaging kidney development in zebrafish. Methods Mol Biol 886:55–70. doi:10.1007/978-1-61779-851-1_6
Perner B, Englert C, Bollig F (2007) The Wilms tumor genes wt1a and wt1b control different steps during formation of the zebrafish pronephros. Dev Biol 309:87–96. doi:10.1016/j.ydbio.2007.06.022
Swanhart LM et al (2010) Characterization of an lhx1a transgenic reporter in zebrafish. Int J Dev Biol 54:731–736. doi:10.1387/ijdb.092969ls
White RM et al (2008) Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2:183–189. doi:10.1016/j.stem.2007.11.002
Drummond IA et al (1998) Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development 125:4655–4667
Sun Z et al (2004) A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development 131:4085–4093. doi:10.1242/dev.01240dev.01240
Mudumana SP, Hentschel D, Liu Y, Vasilyev A, Drummond IA (2008) Odd skipped related 1 reveals a novel role for endoderm in regulating kidney versus vascular cell fate. Development 135:3355–3367. doi:10.1242/dev.022830
de Groh ED et al (2010) Inhibition of histone deacetylase expands the renal progenitor cell population. J Am Soc Nephrol 21:794–802. doi:10.1681/ASN.2009080851
Novitskaya T et al (2013) A PTBA class small molecule enhances recovery and reduces post injury fibrosis after aristolochic acid-induced kidney injury. Am J Physiol Renal Physiol. doi:10.1152/ajprenal.00534.2013
Cianciolo Cosentino C et al (2013) Histone deacetylase inhibitor enhances recovery after AKI. J Am Soc Nephrol 24:943–953. doi:10.1681/ASN.2012111055
Westhoff JH et al (2013) Development of an automated imaging pipeline for the analysis of the zebrafish larval kidney. PLoS ONE 8:e82137. doi:10.1371/journal.pone.0082137
Vogt A, Codore H, Day BW, Hukriede NA, Tsang M (2010) Development of automated imaging and analysis for zebrafish chemical screens. J Vis Exp. doi:10.3791/1900
Sanker S et al (2013) Development of high-content assays for kidney progenitor cell expansion in transgenic zebrafish. J Biomol Screen 18:1193–1202. doi:10.1177/1087057113495296
Villefranc JA, Amigo J, Lawson ND (2007) Gateway compatible vectors for analysis of gene function in the zebrafish. Dev Dyn 236:3077–3087. doi:10.1002/dvdy.21354
Kwan KM et al (2007) The Tol2kit: a multisite gateway-based construction kit for Tol2 transposon transgenesis constructs. Dev Dyn 236:3088–3099. doi:10.1002/dvdy.21343
Kikuta H, Kawakami K (2009) Transient and stable transgenesis using tol2 transposon vectors. Methods Mol Biol 546:69–84. doi:10.1007/978-1-60327-977-2_5
Mosimann C et al (2011) Ubiquitous transgene expression and Cre-based recombination driven by the ubiquitin promoter in zebrafish. Development 138:169–177. doi:10.1242/dev.059345
Chen CH, Durand E, Wang J, Zon LI, Poss KD (2013) Zebraflash transgenic lines for in vivo bioluminescence imaging of stem cells and regeneration in adult zebrafish. Development 140:4988–4997. doi:10.1242/dev.102053
Livet J et al (2007) Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450:56–62. doi:10.1038/nature06293
Pan YA, Livet J, Sanes JR, Lichtman JW, Schier AF (2011) Multicolor Brainbow imaging in zebrafish. Cold Spring Harb Protoc 2011:5546
Pan YA et al (2013) Zebrabow: multispectral cell labeling for cell tracing and lineage analysis in zebrafish. Development 140:2835–2846. doi:10.1242/dev.094631
Cade L et al (2012) Highly efficient generation of heritable zebrafish gene mutations using homo- and heterodimeric TALENs. Nucleic Acids Res 40:8001–8010. doi:10.1093/nar/gks518
Bedell VM et al (2012) In vivo genome editing using a high-efficiency TALEN system. Nature 491:114–118. doi:10.1038/nature11537
Dahlem TJ et al (2012) Simple methods for generating and detecting locus-specific mutations induced with TALENs in the zebrafish genome. PLoS Genet 8:e1002861. doi:10.1371/journal.pgen.1002861
Moore FE et al (2012) Improved somatic mutagenesis in zebrafish using transcription activator-like effector nucleases (TALENs). PLoS ONE 7:e37877. doi:10.1371/journal.pone.0037877
Mali P et al (2013) RNA-guided human genome engineering via Cas9. Science 339:823–826. doi:10.1126/science.1232033
Hwang WY et al (2013) Heritable and precise zebrafish genome editing using a CRISPR-Cas system. PLoS ONE 8:e68708. doi:10.1371/journal.pone.0068708
Hruscha A et al (2013) Efficient CRISPR/Cas9 genome editing with low off-target effects in zebrafish. Development 140:4982–4987. doi:10.1242/dev.099085
Ucero AC et al (2013) Unilateral ureteral obstruction: beyond obstruction. Int Urol Nephrol. doi:10.1007/s11255-013-0520-1
Huen SC, Cantley LG (2014) Macrophage-mediated injury and repair after ischemic kidney injury. Pediatr Nephrol. doi:10.1007/s00467-013-2726-y
Hellman NE et al (2010) The zebrafish foxj1a transcription factor regulates cilia function in response to injury and epithelial stretch. Proc Natl Acad Sci USA 107:18499–18504. doi:10.1073/pnas.1005998107
Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ (2011) New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int 79:33–45. doi:10.1038/ki.2010.337
Hentschel DM et al (2005) Acute renal failure in zebrafish: a novel system to study a complex disease. Am J Physiol Renal Physiol 288:F923–F929. doi:10.1152/ajprenal.00386.2004
Curado S et al (2007) Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies. Dev Dyn 236:1025–1035. doi:10.1002/dvdy.21100
Huang J et al (2013) A zebrafish model of conditional targeted podocyte ablation and regeneration. Kidney Int 83:1193–1200. doi:10.1038/ki.2013.6
• Zhou W, Hildebrandt F (2012) Inducible podocyte injury and proteinuria in transgenic zebrafish. J Am Soc Nephrol 23:1039–1047. This paper uses genetic methods to reversibly injure podocytes in the adult fish and assay for proteinuria
Kramer-Zucker AG, Wiessner S, Jensen AM, Drummond IA (2005) Organization of the pronephric filtration apparatus in zebrafish requires Nephrin, Podocin and the FERM domain protein Mosaic eyes. Dev Biol 285:316–329. doi:10.1016/j.ydbio.2005.06.038
Humphreys BD (2014) Kidney structures differentiated from stem cells. Nat Cell Biol 16:19–21. doi:10.1038/ncb2904
Mae S et al (2013) Monitoring and robust induction of nephrogenic intermediate mesoderm from human pluripotent stem cells. Nat Commun 4:1367. doi:10.1038/ncomms2378
Xia Y et al (2013) Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nat Cell Biol 15:1507–1515. doi:10.1038/ncb2872
Takasato M et al (2014) Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol 16:118–126. doi:10.1038/ncb2894
Lam AQ et al (2013) Rapid and efficient differentiation of human pluripotent stem cells into intermediate mesoderm that forms tubules expressing kidney proximal tubular markers. J Am Soc Nephrol. doi:10.1681/ASN.2013080831
Taguchi A et al (2014) Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14:53–67. doi:10.1016/j.stem.2013.11.010
Song JJ et al (2013) Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med 19:646–651. doi:10.1038/nm.3154
Acknowledgments
This work was supported by NIH Grant F32 DK091998 to CNK; NIH Grant RO1 DK041071 and Harvard Stem Cell Institute Grant D001229 to IAD. Thanks to T. Gallegos for critical reading of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamei, C.N., Drummond, I.A. Zebrafish as a Model for Studying Kidney Regeneration. Curr Pathobiol Rep 2, 53–59 (2014). https://doi.org/10.1007/s40139-014-0044-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40139-014-0044-0