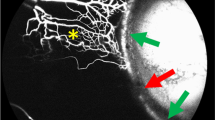
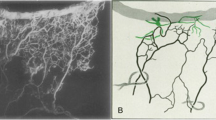
Abstract
Primary open-angle glaucoma (POAG) is the second leading cause of blindness in the world’s rapidly aging population. POAG is characterized by progressive degeneration of neural structures in the posterior segment, often associated with a concomitant elevation of intraocular pressure (IOP). Changes in IOP are believed to be caused by a disruption in the normal outflow of aqueous humor (AH). This article reviews recent research associated with normal and POAG AH outflow. Novel findings elucidating biochemical and pathological changes in the ocular tissues affected in POAG are presented. Stem cell research, identification of lymphatic markers, and increased use of mouse models give researchers exciting new tools to understand AH outflow, changes associated with POAG, and identify underlying causes of the disease.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7.
Overby DR, Stamer WD, Johnson M. The changing paradigm of outflow resistance generation: towards synergistic models of the JCT and inner wall endothelium. Exp Eye Res. 2009;88(4):656–70.
Grant WM. Further studies on facility of flow through the trabecular meshwork. AMA Arch Ophthalmol. 1958;60(4 Part 1):523–33.
Ethier CR, et al. Two pore types in the inner-wall endothelium of Schlemm’s canal. Invest Ophthalmol Vis Sci. 1998;39:2041–8.
Dvorak-Theobald G. Further studies on the canal of Schlemm. Its anastomoses and anatomic relations. Am J Ophthalmol. 1955;39:65–89.
Rohen JW, Rentsch FJ. Uber den Bau des Schlemmschen Kanals und seiner AbfluBwege beim Menschen. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1968;176:309–29.
Johnstone MA. The aqueous outflow system as a mechanical pump: evidence from examination of tissue and aqueous movement in human and non-human primates. J Glaucoma. 2004;13(5):421–38.
Johnstone M, Martin E, Jamil A. Pulsatile flow into the aqueous veins: manifestations in normal and glaucomatous eyes. Exp Eye Res. 2011;92(5):318–27.
Coleman DJ, Trokel S. Direct-recorded intraocular pressure variations in a human subject. Arch Ophthalmol. 1969;82(5):637–40.
Johnstone MA, Grant WG. Pressure-dependent changes in structures of the aqueous outflow system of human and monkey eyes. Am J Ophthalmol. 1973;75(3):365–83.
Grierson I, Lee WR. Changes in the monkey outflow apparatus at graded levels of intraocular pressure: a qualitative analysis by light microscopy and scanning electron microscopy. Exp Eye Res. 1974;19(1):21–33.
Johnson M, et al. The pore density in the inner wall endothelium of Schlemm’s canal of glaucomatous eyes. Invest Ophthalmol Vis Sci. 2002;43:2950–5.
•• Hann CR, et al. Anatomic changes in Schlemm’s canal and collector channels in normal and primary open-angle glaucoma eyes using low and high perfusion pressures. Invest Ophthalmol Vis Sci. 2014;55(9):5834–41. A possible compensatory mechanism for elevated pressure may be found in increased numbers of collector channels in normal and glaucoma eyes.
•• Yang CY, et al. Endothelial glycocalyx layer in the aqueous outflow pathway of bovine and human eyes. Exp Eye Res. 2014;128:27–33. Finding a glycocalyx on Schlemm’s canal and collector channel endothelial surfaces enlarges the biosensory role these cells may play in the outflow pathway.
Tarbell JM, Ebong EE. The endothelial glycocalyx: a mechano-sensor and -transducer. Sci Signal. 2008;1(40):1–5.
Alm A. Uveoscleral outflow—a review. Exp Eye Res. 2009;88(4):760–8.
Alvarado J, et al. Age-related changes in trabecular meshwork cellularity. Invest Ophthalmol Vis Sci. 1981;21:714–27.
Teng CC, Paton RT, Katzin HM. Primary degeneration in the vicinity of the chamber angle; as an etiologic factor in wide-angle glaucoma. Am J Ophthalmol. 1955;40(5 Part 1):619–31.
Johnson M, et al. The pore density in the inner wall endothelium of Schlemm’s canal of glaucomatous eyes. Invest Ophthalmol Vis Sci. 2002;43(9):2950–5.
Allingham RR, de Kater AW, Ethier CR. Schlemm’s canal and primary open angle glaucoma: correlation between Schlemm’s canal dimensions and outflow facility. Exp Eye Res. 1996;62(1):101–9.
Chi HH, Katzin HM, Teng CC. Primary degeneration in the vicinity of the chamber angle; as an etiologic factor in wide-angle glaucoma. II. Am J Ophthalmol. 1957;43(2):193–203.
Tamm S, Tamm E, Rohen JW. Age-related changes of the human ciliary muscle. A quantitative morphometric study. Mech Ageing Dev. 1992;62(2):209–21.
Acott TS, Kelley MJ. Extracellular matrix in the trabecular meshwork. Exp Eye Res. 2008;86(4):543–61.
•• Keller KE, et al. Segmental versican expression in the trabecular meshwork and involvement in outflow facility. Invest Ophthalmol Vis Sci. 2011;52(8):5049–57. The segmental distribution of versican and its effect on outflow may partially explain the segmental nature of aqueous outflow.
Keller KE, et al. Effects of modifiers of glycosaminoglycan biosynthesis on outflow facility in perfusion culture. Invest Ophthalmol Vis Sci. 2008;49(6):2495–505.
Knepper PA, Goossens W, Palmberg PF. Glycosaminoglycan stratification of the juxtacanalicular tissue in normal and primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 1996;37(12):2414–25.
Bradley J, Vranka J, Colvis CM, Conger DM, Alexander JP, Fisk AS, Samples JR, Acott TS. Effect of matrix metalloproteinases activity on outflow in perfused human organ culture. Invest Ophthalmol Vis Sci. 1998;39(13):2649–58.
Aga M, et al. Differential effects of caveolin-1 and -2 knockdown on aqueous outflow and altered extracellular matrix turnover in caveolin-silenced trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2014;55(9):5497–509.
Wiggs JL, et al. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma in Caucasians from the USA. Hum Mol Genet. 2011;20(23):4707–13.
Oh DJ, et al. Overexpression of SPARC in human trabecular meshwork increases intraocular pressure and alters extracellular matrix. Invest Ophthalmol Vis Sci. 2013;54(5):3309–19.
Swaminathan SS, et al. TGF-beta2-mediated ocular hypertension is attenuated in SPARC-null mice. Invest Ophthalmol Vis Sci. 2014;55(7):4084–97.
Fuchshofer R, et al. Bone morphogenetic protein-7 is an antagonist of transforming growth factor-beta2 in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2007;48(2):715–26.
Chowdhury UR, et al. Expression profile of the matricellular protein osteopontin in primary open-angle glaucoma and the normal human eye. Invest Ophthalmol Vis Sci. 2011;52(9):6443–51.
Chatterjee A, et al. The role of SPARC in trabecular meshwork extracellular matrix turnover and IOP regulation. Glaucoma Today; 2012. October, p. 12–5.
Fuchshofer R, Tamm ER. Modulation of extracellular matrix turnover in the trabecular meshwork. Exp Eye Res. 2009;88(4):683–8.
Villarreal G Jr, et al. Canonical wnt signaling regulates extracellular matrix expression in the trabecular meshwork. Invest Ophthalmol Vis Sci. 2014;55(11):7433–40.
Chatterjee A, et al. AMP-activated protein kinase regulates intraocular pressure, extracellular matrix, and cytoskeleton in trabecular meshwork. Invest Ophthalmol Vis Sci. 2014;55(5):3127–39.
Braakman ST, et al. Biomechanical strain as a trigger for pore formation in Schlemm’s canal endothelial cells. Exp Eye Res. 2014;127:224–35.
Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69(1):11–25.
Ramage L. Integrins and extracellular matrix in mechanotransduction. Cell Health Cytoskelet. 2012;4:1–9.
Wolfenson H, Lavelin I, Geiger B. Dynamic regulation of the structure and functions of integrin adhesions. Dev Cell. 2013;24(5):447–58.
Tervo K, et al. Integrins in human anterior chamber angle. Graefes Arch Clin Exp Ophthalmol. 1995;233(5):291–5.
Zhou L, et al. Expression of integrin receptors in the human trabecular meshwork. Curr Eye Res. 1999;19(5):395–402.
Schwinn MK, et al. Heparin II domain of fibronectin mediates contractility through an alpha4beta1 co-signaling pathway. Exp Cell Res. 2010;316(9):1500–12.
Filla MS, et al. regulation of cross-linked actin network (CLAN) formation in human trabecular meshwork (HTM) cells by convergence of distinct β1 and β3 integrin pathways. Invest Ophthalmol Vis Sci. 2009;50(12):5723–31.
Tian B, et al. Cytoskeletal involvement in the regulation of aqueous humor outflow. Invest Ophthalmol Vis Sci. 2000;41:619–23.
Last JA, et al. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Invest Ophthalmol Vis Sci. 2011;52(5):2147–52.
•• Overby DR, et al. The structure of the trabecular meshwork, its connections to the ciliary muscle, and the effect of pilocarpine on outflow facility in mice. Invest Ophthalmol Vis Sci. 2014;55(6):3727–36. Similarities between the mouse and human outflow pathways, particularly in response to pilocarpine and in the structure of the ciliary muscle, lend further strength to use of the mouse as a model for POAG research.
Junglas B, et al. Connective tissue growth factor causes glaucoma by modifying the actin cytoskeleton of the trabecular meshwork. Am J Pathol. 2012;180(6):2386–403.
Overby DR, et al. Altered mechanobiology of Schlemm’s canal endothelial cells in glaucoma. Proc Natl Acad Sci. 2014;111(38):13876–81.
Zhou EH, et al. Mechanical responsiveness of the endothelial cell of Schlemm’s canal: scope, variability and its potential role in controlling aqueous humour outflow. J R Soc Interface. 2012;9(71):1144–55.
Stamer WD, et al. eNOS, a pressure-dependent regulator of intraocular pressure. Invest Ophthalmol Vis Sci. 2011;52(13):9438–44.
Lei Y, et al. Endothelial nitric oxide synthase-related mechanotransduction changes in aged porcine angular aqueous plexus cells. Invest Ophthalmol Vis Sci. 2014;55(12):8402–8.
Alvarado J, Murphy C, Juster R. Trabecular meshwork cellularity in POAG and nonglaucomatous normals. Ophthalmology. 1984;91:564–79.
Du Y, et al. Multipotent stem cells from trabecular meshwork become phagocytic TM cells. Invest Ophthalmol Vis Sci. 2012;53(3):1566–75.
Braunger BM, et al. Identification of adult stem cells in Schwalbe’s line region of the primate eye. Invest Ophthalmol Vis Sci. 2014;55(11):7499–507.
Acott TS, et al. Trabecular repopulation by anterior trabecular meshwork cells after laser trabeculoplasty. Am J Ophthalmol. 1989;107(1):1–6.
•• Ding QJ, et al. Induction of trabecular meshwork cells from induced pluripotent stem cells. Invest Ophthalmol Vis Sci. 2014;55(11):7065–72. The induction of multipotent stem cells to become phagocytic TM cells could lead to future use of cell-based therapies to treat POAG.
Thiery JP, et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–90.
Takahashi E, et al. Epithelial mesenchymal transition-like phenomenon in trabecular meshwork cells. Exp Eye Res. 2014;118:72–9.
Lei Y, et al. Outflow physiology of the mouse eye: pressure dependence and washout. Invest Ophthalmol Vis Sci. 2011;52(3):1865–71.
Li G, et al. Pilocarpine-induced dilation of Schlemm’s canal and prevention of lumen collapse at elevated intraocular pressures in living mice visualized by OCT. Invest Ophthalmol Vis Sci. 2014;55(6):3737–46.
Boussommier-Calleja A, Overby DR. The influence of genetic background on conventional outflow facility in mice. Invest Ophthalmol Vis Sci. 2013;54(13):8251–8.
Millar JC, Clark AF, Pang IH. Assessment of aqueous humor dynamics in the mouse by a novel method of constant-flow infusion. Invest Ophthalmol Vis Sci. 2011;52(2):685–94.
Anderson MG, et al. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat Genet. 2002;30(1):81–5.
Aihara M, Lindsey JD, Weinreb RN. Aqueous humor dynamics in mice. Invest Ophthalmol Vis Sci. 2003;44:5168–73.
Zhou Y, Grinchuk O, Tomarev SI. Transgenic mice expressing the Tyr437His mutant of human myocilin protein develop glaucoma. Invest Ophthalmol Vis Sci. 2008;49:1932–9.
Guorong L, et al. Disease progression in iridocorneal angle tissues of BMP2-induced ocular hypertensive mice with optical coherence tomography. Mol Vis. 2014;20:1695–709.
Ramos RF, et al. Schlemm’s canal endothelia, lymphatic, or blood vasculature? J Glaucoma. 2007;16(4):391–405.
Yucel YH, et al. Identification of lymphatics in the ciliary body of the human eye: a novel “uveolymphatic” outflow pathway. Exp Eye Res. 2009;89(5):810–9.
•• Park DY, et al. Lymphatic regulator PROX1 determines Schlemm’s canal integrity and identity. J Clin Invest. 2014;124(9):3960–74. Identification of the lymphatic regulator PROX1 and its role in the development of Schlemm’s canal adds new evidence supporting a possible lymphatic system in the conventional outflow pathway.
Aspelund A, et al. The Schlemm’s canal is a VEGF-C/VEGFR-3-responsive lymphatic-like vessel. J Clin Invest. 2014;124(9):3975–86.
Bradley JM, et al. Effects of mechanical stretching on trabecular matrix metalloproteinases. Invest Ophthalmol Vis Sci. 2001;42(7):1505–13.
•• Acott TS, et al. Intraocular pressure homeostasis: maintaining balance in a high-pressure environment. J Ocul Pharmacol Ther. 2014;30(2–3):94–101. An excellent review of intraocular homeostasis and the factors that influence it.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Diagnosis and Monitoring of Glaucoma.
Rights and permissions
About this article
Cite this article
Hann, C.R., Fautsch, M.P. Recent Developments in Understanding the Role of Aqueous Humor Outflow in Normal and Primary Open Angle Glaucoma. Curr Ophthalmol Rep 3, 67–73 (2015). https://doi.org/10.1007/s40135-015-0072-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40135-015-0072-x