Abstract
Introduction
Anti-vascular endothelial growth factor (anti-VEGF) injection was widely used in patients with neovascular age-related macular degeneration (nAMD) and polypoidal choroidal vasculopathy (PCV); however, the systemic and local levels of vascular endothelial growth factor (VEGF)-B were seldom detected before. This study was conducted to detect and compare the aqueous humor and plasma VEGF-B levels in nAMD and PCV before and after anti-VEGF therapy.
Methods
Concentrations of VEGF-B in aqueous humor and plasma of individuals with nAMD (n = 10), PCV (n = 22), and age-related cataract controls (n = 12) were measured by enzyme-linked immunosorbent assay. Ranibizumab was injected intravitreally in patients monthly for three consecutive months. Before each injection in patients and at the baseline of controls, blood and aqueous humor samples were collected. Best-corrected visual acuity (BCVA) and central retinal thickness (CRT) were collected before each injection in patient groups. The differences of BCVA, CRT, and VEGF-B levels in aqueous humor and plasma between groups before and after anti-VEGF therapy were compared.
Results
VEGF-B was overexpressed in aqueous humor and plasma of nAMD and PCV groups compared with control group (P < 0.05), but no statistically significant difference existed across nAMD and PCV groups (P > 0.05). Moreover, there were no obvious difference in levels of VEGF-B in aqueous humor and plasma within the treatment groups after anti-VEGF treatment (P > 0.05). The mean CRT in the nAMD group was thinner than that in the PCV group at baseline (P < 0.01). After injections, the CRT obviously declined in both groups (P < 0.05). There was no correlation between CRT reduction and high VEGF-B expression in aqueous humor and plasma of treatment groups.
Conclusion
Overexpression of VEGF-B locally and systemically in patients with nAMD and PCV indicated that elevated VEGF-B concentrations were relevant to the disease processes. Ranibizumab did not influence the levels of VEGF-B in the real world. CRT might help to distinguish PCV from nAMD.
Similar content being viewed by others
Why carry out this study? |
The functions of vascular endothelial growth factor (VEGF)-B are confusing, with some benefits like neuroprotection, anti-apoptotic, and antioxidant activity that may have therapeutic potential in treating patients with polypoidal choroidal vasculopathy (PCV) and neovascular age-related macular degeneration (nAMD), in whom VEGF-B antagonists have been widely used. However, as far as we know, the VEGF-B levels and therapeutic response in plasma and aqueous humor of patients with nAMD and PCV are not clear. |
The study was conducted to detect and compare the aqueous humor and plasma VEGF-B levels in nAMD and PCV before and after anti-VEGF therapy. |
What was learned from the study? |
We found overexpression of VEGF-B in aqueous humor and plasma of patients with nAMD and PCV without response to anti-VEGF therapy, indicating that elevated VEGF-B concentrations were relevant to the disease processes. |
Better knowledge about VEGF-B concentrations may contribute to identification of better druggable candidates for anti-VEGF therapy, but considering the complexity of the function of VEGF-B, the choice of therapy still needs a better understanding of the roles played by VEGF-B in the pathogenic processes of nAMD and PCV in the future. Ranibizumab did not influence the levels of VEGF-B in the real world. CRT might help to distinguish PCV from nAMD. |
Introduction
Age-related macular degeneration (AMD) is a refractory fundus disease threatening the vision of the global aging population. Neovascular AMD (nAMD) involves new vessels growing into the retinal neurepithelium layer from the choroid capillary through Bruch’s membrane/retinal pigment epithelium [1, 2]. nAMD usually rapidly progresses, leading to distortion and disorder of shape, resulting in more cases of legal blindness. Polypoidal choroidal angiopathy (PCV), frequently found in the Asian population, has similarities and differences in clinical manifestations and response to therapy when compared with nAMD [3]. It is controversial whether PCV is a disease independent of nAMD or not. The major step contributing to the pathogenesis of both diseases is overexpression of the cytokine vascular endothelial growth factor (VEGF), which activates choroidal neovascularization and leads to the accumulation of subretinal fluid. Therefore, VEGF has become a major therapeutic target of some molecules for nAMD and PCV [4].
VEGF-A, the main target of anti-VEGF agents, not only acts as an angiogenic cytokine but also as a medium for vascular permeability and angiogenesis in some inflammatory related diseases. At present, several intravitreal anti-VEGF injections have been widely performed in clinical treatment particularly in nAMD and PCV to inhibit neovessel formation and edema. The first approved monoclonal antibody fragment, ranibizumab, aimed to block human VEGF-A; in contrast, aflibercept and conbercept were fusion proteins that combined with VEGF-A, VEGF-B, and placental growth factor. In spite of the beneficial effects, VEGF-A inhibitions had been reported to be associated with geographic atrophy progress, retinal pigment epithelium degeneration, and subsequent retinal neuronal cell apoptosis [5]. Moreover, a quick decrease of the retinal nerve fiber layer thickness had already been observed in patients with nAMD or diabetic macular edema combined with glaucoma undergoing anti-VEGF treatment, demonstrating that VEGF blockage was detrimental to retinal ganglion cell (RGC) survival, especially in individuals already with compromised RGCs [6,7,8].
However, although VEGF-B is similar to VEGF-A in structure, and was discovered decades ago, the physiological role remains ambiguous thus far. A recent report identified that VEGF-B has a gliotrophic effect on Müller cells under pathological circumstances, thus neutralizing the cytokine that might otherwise promote disease progression [9]. Moreover, previous studies highlighted that an essential feature of VEGF-B was to protect retinal cells against apoptosis caused by anti-VEGF-A antagonists [10, 11]. Furthermore, VEGF-B was identified to have antioxidant function mediated by VEGFR1 [12, 13]. VEGFB gene deletion in mice led to degeneration of retina, and supplementation with VEGF-B in the retinitis pigmentosa model rescued retinal cells from death. Considering that oxidative stress was a key factor in numerous diseases, VEGF-B was expected to play a therapeutic role in their treatment [12]. Thus, VEGF-B was believed to be a prospective protective cytokine, which might be of great value in treating retinal diseases related to oxidative stress including nAMD and PCV. Notwithstanding this, therapeutic regimens with neutralization of VEGF-B are widely used in patients with neovascular diseases. Therefore, exploring the levels and function of VEGF-B is important for the selection of anti-VEGF drugs in treating nAMD and PCV. The purpose of present study was to detect VEGF-B concentrations in aqueous humor and plasma samples from patients with nAMD and PCV, to identify the difference between these two diseases from the aspect of cytokine VEGF-B, and to observe the response to intravitreal injection of ranibizumab (IVR).
Methods
Participants and Ethical Approval
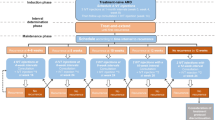
This prospective case–control study included 10 patients with nAMD and 22 with PCV who were treatment-naïve as well as 12 participants with cataracts that underwent surgery and served as the controls. Patients were included following diagnosis and classification of nAMD and PCV by two retinal specialists after examining ocular fundus, fluorescence angiography, and indocyanine green angiography. The exclusion criteria were related ocular diseases like glaucoma, pathological myopia, uveitis, choroiditis, any types of retinal diseases, vitreous hemorrhage, hereditary diseases and ocular trauma, with a history of intraocular surgery and systemic immune dysfunction, treatment with systemic anti-inflammatory therapy for any reason, and diabetes mellitus.
The study involving human participants was in accordance with the tenets of the Declaration of Helsinki and was reviewed and approved by the Ethics Committee of Peking Union Medical College Hospital (Beijing, China) with reference number ZS-1125. After receiving an explanation of the possible consequences of the study, all participants signed informed consent to participate before participation.
Protocol and Data Collection
The disease groups received a loading dose (0.05 mg/0.05 ml) of three consecutive IVR (Lucentis®) every 4 weeks, and before each treatment, best-corrected visual acuity (BCVA) and central retinal thickness (CRT) were collected. Blood and aqueous samples were collected prior to each intravitreal injection in treatment groups and before cataract surgery in the control group. Blood samples were drawn into lithium heparin stabilized tubes to prevent platelet activation, centrifugated for 10 min at 3000 rpm at 4 °C, and 0.1 ml of aqueous humor was drawn from the anterior chamber. All collected specimens were frozen at − 80 °C for final quantification. Aqueous humor and plasma VEGF-B concentrations were measured by enzyme-linked immunosorbent assay (human VEGF-B ELISA Kit; Raybiotech, USA) as per the manufacturer’s instructions. The samples involved were analyzed together in duplicate.
Statistical Analyses
All data were analyzed by using the Statistical Package for the Social Sciences statistical software for Windows, version 16.0 (SPSS Inc.). BCVA was converted into logarithm of the minimum angle of resolution (logMAR) for statistical analysis. We conducted the Kolmogorov–Smirnov test to evaluate all variables for normal distribution. One-way analysis of variance (ANOVA) was used for normally distributed continuous variables and the nonparametric Mann–Whitney U test was used for nonnormally distributed continuous variables when comparing characteristics among the three groups. Two-related-samples test or paired-samples T test was applied to make comparisons with changes of VEGF-B concentrations before and after therapy within treatment groups depending on normality assumptions for continuous variables. The correlations were assessed by Pearson or Spearman correlation because of the normality assumption underlying linear regression. A P value less than 0.05 was considered to be statistically significant.
Results
Baseline characteristics of the 44 participants in the analysis are listed in Table 1.
Age distribution did not differ significantly (nAMD = 72.0 ± 8.58 (mean ± standard deviation), PCV = 70.14 ± 7.83, controls = 69.17 ± 12.90 years old, respectively, all P > 0.05) (Table 1). The male to female ratios in the nAMD group, PCV group, and control group were 5:5, 17:5, and 5:7, respectively. No statistical difference was shown in gender distribution among the three groups (Table 1). At baseline, the average values of BCVA were 1.00 ± 0.56 logMAR in the nAMD group and 0.74 ± 0.38 logMAR in the PCV group (P > 0.05) (Fig. 1). After the second IVR, the mean BCVA values in the nAMD and PCV groups were 0.74 ± 0.57 logMAR and 0.56 ± 0.30 logMAR, respectively (P > 0.05). The BCVA changes after the second IVR in the nAMD and PCV group were − 0.26 ± 0.40 logMAR and − 0.18 ± 0.24 logMAR, respectively (P > 0.05). Although BCVA improved after treatment, no statistically significant difference was noted in both groups (Fig. 1).
BCVA before and after anti-VEGF therapy in the nAMD and PCV groups. At baseline, the average values of BCVA were 1.00 ± 0.56 logMAR in the nAMD group, 0.74 ± 0.38 logMAR in the PCV group (P > 0.05). After the 2nd IVR, the mean BCVA in the nAMD and PCV groups were 0.74 ± 0.57 logMAR and 0.56 ± 0.30 logMAR, respectively (P > 0.05). The BCVA changes after treatment in the nAMD and PCV group were − 0.26 ± 0.40 logMAR and − 0.18 ± 0.24 logMAR, respectively (P > 0.05). BCVA best-corrected visual acuity, logMAR logarithm of the minimum angle of resolution, BL baseline, 2nd IVR second intravitreal injection ranibizumab, nAMD neovascular age-related macular degeneration, PCV polypoidal choroidal angiopathy
The average value of CRT at baseline in the nAMD group was 298.90 ± 101.12 μm, whereas in the PCV group it was 402.14 ± 125.27 μm, and significant difference existed within the two groups (P = 0.03) (Fig. 2). After the second IVR, the CRT in the nAMD group was 182.60 ± 81.89 μm; however, in the PCV group it was 296.41 ± 141.18 μm, with significant statistical difference between the groups (P = 0.025). The CRT decreased significantly in both groups after the second injection compared with baseline: in the nAMD group the change was – 116.30 ± 105.69 μm (P = 0.007) and in the PCV group it was − 105.73 ± 102.19 μm (P < 0.0001), with statistical difference (Fig. 2).
CRT before and after anti-VEGF therapy in the nAMD and PCV groups. The mean CRT at baseline in the nAMD group was 298.90 ± 101.12 μm, while in the PCV group it was 402.14 ± 125.27 μm, and significant statistical difference existed within the two groups (P = 0.03). After the 2nd IVR, the CRT in the nAMD group was 182.60 ± 81.89 μm and in the PCV group it was 296.41 ± 141.18 μm, with statistical difference (P = 0.025). The CRT decreased significantly in both groups after the 2nd IVR compared with baseline: in the nAMD group the change was − 116.30 ± 105.69 μm (P = 0.007) and in the PCV group it was − 105.73 ± 102.19 μm (P < 0.0001). CRT central retinal thickness, BL baseline, 2nd IVR second intravitreal injection ranibizumab, nAMD neovascular age-related macular degeneration, PCV polypoidal choroidal angiopathy
The plasma VEGF-B baseline levels in the nAMD, PCV, and control groups were 6.10 ± 6.26, 5.30 ± 6.58, and 1.01 ± 3.50 ng/mL, respectively (P < 0.05). Distinct differences were revealed among all groups. The baseline levels of VEGF-B in plasma were significantly upregulated in the nAMD and PCV groups in contrast to that in the control group (P = 0.017, P = 0.028, respectively). Nevertheless, no apparent difference existed between the nAMD and PCV groups (P = 0.675) (Table1).
Compared with the baseline levels, plasma VEGF-B levels in the nAMD group reduced to 3.75 ± 6.18 ng/mL after the first IVR whereas they increased to 9.25 ± 7.35 ng/mL after the second IVR without statistical significance (all P > 0.05). Similarly, in the PCV group, VEGF-B baseline levels reduced to 4.11 ± 5.46 ng/mL after the first injection while they went back to the baseline levels, 5.36 ± 6.94 ng/mL after the second injection (all P > 0.05) (Fig. 3).
Plasma VEGF-B levels before and after anti-VEGF therapy in the nAMD and PCV groups. Compared with the baseline levels, plasma VEGF-B levels in patients with nAMD decreased to 3.75 ± 6.18 ng/mL after the 1st IVR whereas they increased to 9.25 ± 7.35 ng/mL after the 2nd IVR. In patients with PCV, VEGF-B baseline levels decreased to 4.11 ± 5.46 ng/mL after the 1st IVR but returned to the baseline levels, 5.36 ± 6.94 ng/mL after the 2nd IVR (all P > 0.05). VEGF vascular endothelial growth factor, BL baseline, 1st IVR first intravitreal injection ranibizumab, 2nd IVR second intravitreal injection ranibizumab, nAMD neovascular age-related macular degeneration, PCV polypoidal choroidal angiopathy
The baseline aqueous humor VEGF-B levels in the nAMD, PCV and control groups were 9.52 ± 7.42, 8.22 ± 7.12, and 3.29 ± 6.05 ng/ml, respectively (P < 0.05). Statistically significant differences were discovered among all groups. The baseline VEGF-B concentrations in aqueous humor of the nAMD and PCV groups were apparently raised in comparison to the control group (P = 0.006, P = 0.028, respectively). On the other hand, no statistically significant difference existed between the nAMD and PCV groups (P = 0.458) (Table1).
In comparison with the baseline levels, aqueous humor VEGF-B concentrations in the nAMD group dropped to 7.40 ± 5.37 ng/mL after the first IVR but recovered to the baseline levels, 10.49 ± 6.17 ng/mL after the second IVR (all P > 0.05). Similarly, VEGF-B levels were downregulated to 6.12 ± 7.06 ng/mL after the first injection and returned to 6.32 ± 6.98 ng/mL after the second injection in the PCV group (all P > 0.05) (Fig. 4).
Aqueous humor VEGF-B levels before and after anti-VEGF therapy in the nAMD and PCV groups. Compared with the baseline levels, aqueous humor VEGF-B levels in patients with nAMD decreased to 7.40 ± 5.37 ng/mL after the 1st IVR but returned to the baseline levels, 10.49 ± 6.17 ng/mL after the 2nd IVR. Similarly, in patients with PCV the levels decreased to 6.12 ± 7.06 ng/mL after the 1st IVR but returned to 6.32 ± 6.98 ng/mL after the 2nd IVR (all P > 0.05). VEGF vascular endothelial growth factor, BL baseline, 1st IVR first intravitreal injection ranibizumab, 2nd IVR second intravitreal injection ranibizumab, nAMD neovascular age-related macular degeneration, PCV polypoidal choroidal angiopathy
At baseline, there were no obvious correlations between BCVA and CRT as well as VEGF-B levels in humor and plasma of nAMD and PCV participants (all P > 0.05). After treatment, strong correlations were identified between BCVA improvement and CRT reduction in the nAMD group (r = 0.633, P = 0.025), whereas there were no similar relationships in the PCV group (r = 0.121, P > 0.05). No apparent relevance between BCVA improvement and VEGF-B overexpression in aqueous humor and plasma of treatment groups was found, and similarly no correlation was shown in CRT reduction.
Discussion
This study was significant for following reasons. (1) Overexpression of aqueous humor and plasma VEGF-B in patients with nAMD and PCV contrasted with controls, as far as we know, which had not been reported before, indicating that VEGF-B might be involved in the process of nAMD and PCV. Therefore, considering the complexity of the function of VEGF-B, the selection of anti-VEGF drugs in the treatment of nAMD and PCV depends on a better understanding of the roles that VEGF-B plays in the pathogenic process. (2) The data further confirmed that ranibizumab did not influence the levels of VEGF-B in the real world. (3) From the perspective of VEGF-B concentrations, no statistically significant difference between nAMD and PCV was found, while CRT in nAMD was thinner than in PCV before and after treatment, so CRT might help to distinguish nAMD from PCV.
The VEGF receptor (VEGFR) family is composed of three cytokines, namely VEGFR1, 2, and 3. The VEGF family consists of five VEGFR ligands, namely VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factor (PlGF) [14]. VEGF-A binds to VEGFR1 and VEGFR2, whereas VEGF-B and PlGF uniquely bind to VEGFR1. Furthermore, VEGF-C and VEGF-D primarily bind with VEGFR3. VEGF-B had the ability to compete with VEGF-A for binding to VEGFR1, releasing more VEGF-A, indirectly resulting in increasing VEGFR2 activation. In other words, in spite of lacking affinity for VEGFR2, VEGF-B is still capable of activating VEGFR2 signaling indirectly [15]. The role of VEGFR1 in nAMD and PCV was suggested to be linked to vascular permeability, pathological neovascularization, and recruitment and activation in inflammation [15,16,17]. VEGFR2 was elucidated to have a key role in angiogenesis and permeability [18, 19]. Therefore, the relationships between VEGF-B and coactions with the VEGFR1 and VEGFR2 receptors is fairly intricate and it resulted in VEGF-B becoming the most elusive cytokine of the VEGF family, because its functions in the eye remained to be fully elucidated.
VEGF-B has a wide tissue distribution and is expressed in various tissues and cells, especially the heart, diaphragm, and skeletal muscle. Therefore there was an urgent need to fully explore the functional roles of VEGF-B. Through analyzing the angiogenic role of VEGF-B under normal and pathologic circumstances, contrasting results were obtained with some studies indicating that VEGF-B was angiogenic and others the opposite [13, 20, 21]. Mesquita et al. [21] stated that VEGF-B had no definite angiogenic effect and hence it did not promote vessel formation or sprouting, whereas its transgenic overexpression minimally increased vasculature, and overexpression of VEGF-B did not lead to any side effects. On the other hand, another study insisted that increasing VEGF-B induced retinal and choroidal neovascularization, and damaged the blood-retinal barrier without producing inflammation when compared to controls, which was associated with the progression of AMD [20]. In addition, since VEGF-B played a crucial role in anti-apoptosis and neuroprotection, retinal neuron cells were protected from apoptosis and degeneration. Another study provided evidence that VEGF-B was a neuroprotective cytokine for injured retinal ganglion neurons; using recombinant VEGF-B, the authors obtained a neuroprotective effect on RGCs through an autocrine/paracrine mechanism [6]. Furthermore, compared with the other VEGF family cytokines, VEGF-B was produced the most by Müller cells. At the same time, VEGF-B protected Müller cells from oxidative and hypoxic damage, although it was not necessary for Müller cell survival in normal environments. In pathologic circumstances, Müller cells produced inflammatory and angiogenic cytokines which resulted in neurovascular dysfunction [9]. For the past few years, VEGF-B was suggested to be a promising antioxidant through upregulating vital antioxidant enzymes. In consequence, one of the main roles of VEGF-B was to prevent oxidative stress-induced damage to tissues and cells. Therefore, VEGF-B might have a potential therapeutic effect on oxidative stress-related diseases [11]. Considering the aforementioned positive effects, the use of VEGF-B inhibitor might induce significant adverse events; hence, ranibizumab was presumed to be a good alternative [22].
Previously, numerous cytokines like VEGF and VEGF-A were generally tested in neovascular diseases, whereas the concentrations of VEGF-B were rarely measured specifically [23,24,25,26,27]. Regardless of some research revealing obvious upregulation of VEGF-B in plasma and vitreous of patients with diabetic retinopathy, VEGF-B concentrations were seldom reported in nAMD and PCV [28,29,30]. This study provided novel information that local and systemic levels of VEGF-B were elevated in treatment groups when compared with the control group at baseline, indicating that the VEGF-B might be related to the disease process of nAMD and PCV. However, the results were inconsistent with a previous report which revealed that the baseline systemic VEGF-B levels were not different in patients with nAMD between aflibercept and the control group [23]. On the basis of these observations, we believe that exploring the function and therapeutic potential of VEGF-B in nAMD and PCV is essential in future studies, which may contribute to identification of better therapeutic druggable candidates for therapy. Moreover, a report documented that systemic VEGF-B levels were not different within and between treatment groups 1 week and 4 weeks after intraocular injection aflibercept, even though aflibercept was aimed to be combined with VEGF-B [23]. These findings concurred with our study which provided data for no apparent reduction in local and systemic levels of VEGF-B after treatment by IVR when compared with the baseline, consistent with the theory that ranibizumab did not change VEGF-B concentrations, which were rarely tested in the real world before [1]. In spite of patients treated with IVR showing a mild decrease after 1 month, the suppression was not continuous and went back to baseline levels after the second injection, and none of the changes were statistically significant. In addition, this article showed no obvious statistical difference in VEGF-B levels between the treatment groups, in line with previous studies despite the detection and analysis in a large amount of different cytokines [24, 31]. Thus, other perspectives to distinguish nAMD from PCV are necessary.
In our study, the BCVA improved after treatment, but no obvious statistical difference existed in both groups. We speculated that it might be limited by the small sample size, and larger sample sizes in further research might amplify statistical differences significantly. In the present study, the decrease in CRT in both groups proved to be effective for the treatment with anti-VEGF drugs, consistent with previous studies [32,33,34]. At the same time, the CRT had significantly statistical difference between the nAMD group and PCV group at baseline, and the CRT in nAMD remained thinner than in PCV after treatment; thus, we hypothesized that CRT might help to distinguish PCV from nAMD. Our study proved that BCVA improvement was strongly correlated with CRT reduction in the nAMD group rather than in PCV group; therefore, nAMD and PCV might have different drug responses to anti-VEGF therapy in accordance with previous studies [35, 36]. Moreover, we analyzed the correlation between BCVA as well as CRT and VEGF-B levels before and after anti-VEGF treatment, but failed to find strong correlations. The reason we supposed was that changes in BCVA and CRT were mainly influenced by anti-VEGF-A drug which did not change the concentrations of VEGF-B. As for whether BCVA and CRT were affected by the changes of VEGF-B concentrations, further studies using anti-VEGF-B monotherapy are required.
There were several limitations of our study. Firstly, the sample size was relatively limited; thus, research with larger sample sizes is needed in the future. Secondly, the drug we used in this study simply inhibited VEGF-A, while the response of the drugs targeting VEGF-B should be tested in the future. Thirdly, the data after treatment was collected before the third injection rather than 1 month after the third injection because some patients did not need a fourth injection and there was a moderate rate of loss to follow-up after the last injection. Longer follow-up is needed in further research.
Conclusion
Local and systemic overexpression of VEGF-B in nAMD and PCV indicated that elevated VEGF-B concentrations were relevant to the disease processes. Ranibizumab indeed did not significantly change the concentrations of VEGF-B in the real world. Moreover, the dysregulation of VEGF-B levels could not help to unravel the differences between the two diseases, whereas CRT might be an identifiable point. Our results may be helpful in the selection of better druggable candidates for anti-VEGF therapy, but considering the complexity of the function of VEGF-B, further research exploring the function and therapeutic potential of VEGF-B in nAMD and PCV is required to help to determine appropriate choice of candidates.
References
Bobadilla M, Pariente A, Oca AI, Pelaez R, Perez-Sala A, Larrayoz IM. Biomarkers as predictive factors of anti-VEGF response. Biomedicines. 2022;10(5):1003.
Fursova AZ, Derbeneva AS, Vasilyeva MS, et al. New findings on pathogenetic mechanisms in the development of age-related macular degeneration. Vestn Oftalmol. 2022;138(2):120–30.
Sahu Y, Chaudhary N, Joshi M, Gandhi A. Idiopathic polypoidal choroidal vasculopathy: a review of literature with clinical update on current management practices. Int Ophthalmol. 2021;41(2):753–65.
Kim S, Min G, Kim B, et al. Novel dual-targeting antibody fragment IDB0062 overcomes anti-vascular endothelial growth factor drug limitations in age-related macular degeneration. Transl Vis Sci Technol. 2021;10(14):35.
Cho HJ, Park SM, Kim J, et al. Progression of macular atrophy in patients undergoing anti-vascular endothelial growth factor therapy for neovascular age-related macular degeneration. Acta Ophthalmol. 2021;99(4):e540–6.
Froger N, Matonti F, Roubeix C, et al. VEGF is an autocrine/paracrine neuroprotective factor for injured retinal ganglion neurons. Sci Rep. 2020;10(1):12409.
Gunay BO, Esenulku CM. Retinal nerve fibre layer and ganglion cell layer thickness changes following intravitreal aflibercept for age-related macular degeneration. Cutan Ocul Toxicol. 2022;41(1):91–7.
Lee SW, Sim HE, Park JY, et al. Changes in inner retinal layer thickness in patients with exudative age-related macular degeneration during treatment with anti-vascular endothelial growth factor. Medicine (Baltimore). 2020;99(17):e19955.
Llorian-Salvador M, Barabas P, Byrne EM, et al. VEGF-B is an autocrine gliotrophic factor for Müller cells under pathologic conditions. Invest Ophthalmol Vis Sci. 2020;61(11):35.
Zhu Y, Zhang Y, Qi X, et al. GAD1 alleviates injury-induced optic neurodegeneration by inhibiting retinal ganglion cell apoptosis. Exp Eye Res. 2022;223:109201.
Chen R, Lee C, Lin X, Zhao C, Li X. Novel function of VEGF-B as an antioxidant and therapeutic implications. Pharmacol Res. 2019;143:33–9.
Arjunan P, Lin X, Tang Z, et al. VEGF-B is a potent antioxidant. Proc Natl Acad Sci U S A. 2018;115(41):10351–6.
Mota F, Yelland T, Hutton JA, et al. Peptides derived from vascular endothelial growth factor B show potent binding to neuropilin-1. ChemBioChem. 2022;23(1):e202100463.
Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176(6):1248–64.
Uemura A, Fruttiger M, D’Amore PA, et al. VEGFR1 signaling in retinal angiogenesis and microinflammation. Prog Retin Eye Res. 2021;84:100954.
Tarallo V, Iaccarino E, Cicatiello V, Sanna R, Ruvo M, De Falco S. Oral delivery of a tetrameric tripeptide inhibitor of VEGFR1 suppresses pathological choroid neovascularization. Int J Mol Sci. 2020;21(2):410.
Motohashi R, Noma H, Yasuda K, Kotake O, Goto H, Shimura M. Dynamics of inflammatory factors in aqueous humor during ranibizumab or aflibercept treatment for age-related macular degeneration. Ophthalmic Res. 2017;58(4):209–16.
Cheng K, Liu CF, Rao GW. Anti-angiogenic agents: a review on vascular endothelial growth factor receptor-2 (VEGFR-2) inhibitors. Curr Med Chem. 2021;28(13):2540–64.
Jeong HS, Yun JH, Lee DH, Lee EH, Cho CH. Retinal pigment epithelium-derived transforming growth factor-beta2 inhibits the angiogenic response of endothelial cells by decreasing vascular endothelial growth factor receptor-2 expression. J Cell Physiol. 2019;234(4):3837–49.
Zhong X, Huang H, Shen J, et al. Vascular endothelial growth factor-B gene transfer exacerbates retinal and choroidal neovascularization and vasopermeability without promoting inflammation. Mol Vis. 2011;17:492–507.
Mesquita J, Castro-de-Sousa JP, Vaz-Pereira S, Neves A, Passarinha LA, Tomaz CT. Vascular endothelial growth factors and placenta growth factor in retinal vasculopathies: current research and future perspectives. Cytokine Growth Factor Rev. 2018;39:102–15.
Woo SJ, Veith M, Hamouz J, et al. Efficacy and safety of a proposed ranibizumab biosimilar product vs a reference ranibizumab product for patients with neovascular age-related macular degeneration: a randomized clinical trial. JAMA Ophthalmol. 2021;139(1):68–76.
Angermann R, Huber AL, Nowosielski Y, et al. Changes in systemic levels of vascular endothelial growth factor after intravitreal injection of aflibercept or brolucizumab for neovascular age-related macular degeneration. Retina. 2022;42(3):503–10.
Zhou H, Zhao X, Yuan M, Chen Y. Comparison of cytokine levels in the aqueous humor of polypoidal choroidal vasculopathy and neovascular age-related macular degeneration patients. BMC Ophthalmol. 2020;20(1):15.
de Freitas LGA, Isaac DLC, Abud MB, et al. Analysis of cytokines in the aqueous humor during intravitreal Ranibizumab treatment of diabetic macular edema. Sci Rep. 2021;11(1):23981.
Liu C, Zhang S, Deng X, et al. Comparison of intraocular cytokine levels of choroidal neovascularization secondary to different retinopathies. Front Med (Lausanne). 2021;8:783178.
Balne PK, Agrawal R, Au VB, et al. Dataset of plasma and aqueous humor cytokine profiles in patients with exudative age related macular degeneration and polypoidal choroidal vasculopathy. Data Brief. 2018;19:1570–3.
Mesquita J, Castro-de-Sousa JP, Vaz-Pereira S, Neves A, Passarinha LA, Tomaz CT. Evaluation of the growth factors VEGF-A and VEGF-B in the vitreous and serum of patients with macular and retinal vascular diseases. Growth Factors. 2018;36(1–2):48–57.
Zhang Y, Gao Z, Zhang X, et al. Effect of intravitreal conbercept injection on VEGF-A and -B levels in the aqueous and vitreous humor of patients with proliferative diabetic retinopathy. Exp Ther Med. 2021;21(4):332.
Zhang X, Wu J, Wu C, Bian AL, Geng S, Dai RP. Comparison of aqueous humor levels of PlGF and VEGF in proliferative diabetic retinopathy before and after intravitreal conbercept injection. Diabetes Res Clin Pract. 2020;162: 108083.
Agrawal R, Balne PK, Wei X, et al. Cytokine profiling in patients with exudative age-related macular degeneration and polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. 2019;60(1):376–82.
Lu Y, Huang W, Zhang Y, et al. Factors for visual acuity improvement after anti-VEGF treatment of wet age-related macular degeneration in China: 12 months follow up. Front Med (Lausanne). 2021;8:735318.
Takahashi K, Iida T, Ishida S, et al. Effectiveness of current treatments for wet age-related macular degeneration in Japan: a systematic review and pooled data analysis. Clin Ophthalmol. 2022;16:531–40.
Fenner BJ, Ting DSW, Tan ACS, et al. Real-world treatment outcomes of age-related macular degeneration and polypoidal choroidal vasculopathy in Asians. Ophthalmol Retina. 2020;4(4):403–14.
Diaz-Villamarin X, Blanquez-Martinez D, Pozo-Agundo A, et al. Genetic variants affecting anti-VEGF drug response in polypoidal choroidal vasculopathy patients: a systematic review and meta-analysis. Genes (Basel). 2020;11(11):1335.
Kokame GT, deCarlo TE, Kaneko KN, Omizo JN, Lian R. Anti-vascular endothelial growth factor resistance in exudative macular degeneration and polypoidal choroidal vasculopathy. Ophthalmol Retina. 2019;3(9):744–52.
Acknowledgements
We want to express our gratitude to Mengmeng Shang and Meng Wang for their help with statistics.
Funding
The authors are thankful for the financial support of Beijing Natural Science Foundation Beijing-Tianjin-Hebei Basic Research Foundation: J200007, including but not limited to the study and the rapid service fee.
Author Contributions
HZ was responsible for the concept, design, acquisition, analysis, interpretation of data and was a major contributor in writing the manuscript. XZ and SW were responsible for the optimization of the manuscript. All authors (HZ, XZ, SW, YC) have been involved in revising and giving the final approval of the version to be published. All authors (HZ, XZ, SW, YC) agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors read and approved the final manuscript.
Disclosures
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Compliance with Ethics Guidelines
The study involving human participants was in accordance with the tenets of the Declaration of Helsinki and was reviewed and approved by the Ethics Committee of Peking Union Medical College Hospital (Beijing, China) with reference number ZS-1125. After receiving an explanation of the possible consequences of the study, all participants signed informed consent to participate before participation.
Data Availability
The datasets supporting the conclusions of this article will be available from the corresponding author on reasonable request.
Thanking Patient Participants
All authors thank all study participants for their involvement in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Zhou, H., Zhao, X., Wang, S. et al. Determination of Vascular Endothelial Growth Factor-B Concentrations in Aqueous Humor and Plasma of Neovascular Age-Related Macular Degeneration and Polypoidal Choroidal Vasculopathy Patients Before and After Anti-VEGF Therapy. Ophthalmol Ther 12, 827–837 (2023). https://doi.org/10.1007/s40123-022-00618-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-022-00618-4