Abstract
Introduction
Little is known of the ocular distribution characteristics of currently branded non-steroidal anti-inflammatory drugs (NSAIDs) in the United States. This study was designed to predict the ocular bioavailability characteristics in humans using Dutch Belted rabbits as a surrogate. Commercially available, topically-applied NSAIDs containing bromfenac or nepafenac/amfenac were evaluated.
Methods
126 healthy adult Dutch Belted rabbits were randomly assigned to three treatment cohorts (BromSite® twice daily [BID] in the right eye, BromSite® once daily [QD] in the right eye, Prolensa® QD in the right eye and Ilevro™ QD in the left eye) and 7 post-dosing time points (0.5, 1, 2, 4, 8, 12, 24 h after final instillation). The study eyes received 40 µL of the assigned drug for a consecutive 9 days. Samples of aqueous humor, iris-ciliary body, choroid, sclera, and retina were harvested from the study eyes at the assigned time point after the last dose on the 9th day. NSAID content in ocular tissues was analyzed using high-performance liquid chromatography (HPLC), and area under the curve (AUC0.5–24h), maximum concentration (Cmax), and time to maximum concentration (Tmax) were determined.
Results
Peak NSAID concentrations were reached within 1–3 h in the anterior segment and within 1–3 h in the posterior segment after last dose. Throughout the ocular tissues, both AUC and Cmax for BromSite® (BID and QD) were consistently higher than respective NSAID concentrations of Prolensa® QD and Ilevro® QD. When comparing BromSite® BID to QD, the BID regimen produced generally higher but statistically similar bromfenac concentrations throughout the ocular tissues except in the aqueous humor and iris-ciliary body, where the AUC BID was statistically significantly higher with BromSite® BID.
Conclusion
As a surrogate to human ocular bioavailability, BromSite® demonstrated significantly greater NSAID compared to Prolensa® QD and Ilevro® QD. The DuraSite® component of BromSite® appears to enhance ocular penetration throughout both anterior and posterior tissues.
Funding
Sun Pharmaceutical Industries Ltd.
Similar content being viewed by others
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) have been a standard of care in ophthalmology for decades. Nepafenac, ketorolac, diclofenac, flurbiprofen, and bromfenac are currently approved by the US Food and Drug Administration (FDA). With different pharmacodynamic characteristics, these NSAIDs inhibit isoforms 1 and 2 of cyclooxygenase (COX). The pharmacology is known to prevent the biosynthesis of inflammatory mediators responsible for inflammation, producing vasodilation, altering intraocular pressure, and miosis [1,2,3,4].
Topical steroids have been the mainstay of therapy to prevent or control ocular inflammation [5]. However, corticosteroids in some patients may raise intraocular pressure, impair wound healing, suppress the immune system, increase infection risk, and can contribute to cataracts [6, 7]. Clinically, NSAIDs are used by ophthalmologists in combination with, or in place of, corticosteroids. For ophthalmic surgical procedures, NSAIDs are useful for mydriasis, analgesia, and anti-inflammatory effects specific to ocular tissue.
The FDA has approved these products in five indications: (1) seasonal allergic conjunctivitis, (2) pain associated with cataract surgery, (3) inflammation associated with cataract surgery, (4) pain associated with corneal refractive surgery, and (5) inhibition of intraoperative miosis. In addition, while not approved by the FDA specifically for this use, NSAIDs are considered the standard of care by many corneal specialists for preventing cystoid macular edema (CME) associated with cataract surgery. Recently, the American Academy of Ophthalmology (AAO) stated in an ophthalmic technology assessment that there is good collective clinical evidence and rationale that anti-inflammatory use beginning 72 h prior to surgery “reduces CME and improves vision in the short term” [8].
In general, from a molecular perspective, halogenation is considered to augment drug therapy strength (i.e., H < F− < Cl− < I− ~ Br−) [9]. For example, amfenac and bromfenac chemical structures are identical except for the C-4 molecular position, where amfenac has a hydrogen and bromfenac has a bromine. It is thought this difference may allow bromfenac greater ocular distribution [10].
Baklayan et al. performed a 24-h analysis of 0.09% commercial bromfenac formulation (Xibrom®) and 14C-labeled bromfenac administered to the ocular surface. This study was performed in rabbits (New Zealand white) [11]. Two studies were conducted: the former study measured available radiolabeled bromfenac. The next study analyzed 0.09% bromfenac ophthalmic solution. The 2 animal cohorts strongly suggested 1 dose topically of 0.09% bromfenac ophthalmic solution achieved quantifiable levels throughout ocular tissues within 120 min, and levels were detected over a 24 h period. Within the discussion, the authors speculate the bromfenac at the C-4 position was the primary factor for enhanced bromfenac penetration.
Si and colleagues conducted the first published studies with bromfenac in DuraSite®. (This was not BromSite® [12].) In a contra-lateral eye design, the authors compared 0.045% and 0.09% bromfenac within a DuraSite® formulation to the commercial product, Xibrom® in rabbits (Dutch Belted). A single, topical ocular dose study and a multi-dose study were reported. Bromfenac tissue concentrations were reported by standard methodology (i.e., HPLC, high-performance liquid chromatography with tandem mass spectrometry).
In the single-dose study, concentrations of bromfenac were measured in the aqueous humor (AH) of 84 rabbits after 1 dose of either 0.09% or 0.045% bromfenac (in DuraSite®) left eye (OS) and the 0.09% commercial preparation (Xibrom®) right eye (OD). AH samples were taken at 0.5, 1, 2, 4, 8, 12, and 24 h after instillation. AH bromfenac was greater with either DuraSite™ formulation compared to Xibrom®. The area-under the curve (AUC) of 0.045% and 0.09% bromfenac in DuraSite was approximately two- and fourfold greater, respectively, than that of Xibrom®.
In the separate multiple-dose study, rabbits were administered 1 drop of the experimental 0.09% DuraSite formulation or 0.09% Xibrom®, three times daily (TID) for 14 days. The ocular tissue concentrations of bromfenac were generally about 3 times higher in the experimental group compared to Xibrom®. It should be noted, however, the tissue concentrations for bromfenac in DuraSite® were significantly higher than those of Xibrom® for all tissues except the retina. In the retina, the bromfenac concentrations were essentially identical: 34.0 ng/g for bromfenac in DuraSite® compared to 32.4 ng/g, for Xibrom®.
BromSite® (bromfenac ophthalmic solution 0.075%) is a newer bromfenac formulation indicated for the treatment of postoperative inflammation and prevention of ocular pain in patients undergoing cataract surgery [13]. The formulation consists of DuraSite®, a mucoadhesive matrix that swells in aqueous media and stabilizes small molecules, contributing to longer ocular surface dwelling times [14].
The ocular pharmacokinetic (PK) details of BromSite® have not been published to date. Additionally, there are no studies to address PK comparisons to either Prolensa® (bromfenac ophthalmic solution 0.07%) or Ilevro® (nepafenac ophthalmic suspension 0.3%). This study was designed to predict the ocular bioavailability characteristics in humans (using Dutch Belted rabbits as a surrogate) of topical ocular NSAIDs containing bromfenac or nepafenac/amfenac. To our knowledge, this study is the first to address both topics.
Methods
A total of 126 healthy adult Dutch Belted rabbits were randomly assigned to 21 groups that formed three treatment cohorts. Each cohort consisted of 7 post-dosing time point groups in size of 6 each with equal distribution in gender. As detailed in Table 1, rabbits in Cohort A received BromSite® BID (twice daily) OD; Cohort B, BromSite® QD (once daily) OD; Cohort C, both eyes (OU) were studied, Prolensa® QD in right eye and Ilevro™ QD in left eye. Because both BromSite® and Prolensa® contain bromfenac, these two products were not administered to the same animal (to avoid possible cross-over contamination error).
On the morning of Day 9, all animals received their last dose and then humanely euthanatized at the assigned time points. Following euthanasia, each study eye was enucleated and immediately flash frozen in liquid N2. Later, ocular tissues including the aqueous humor, iris-ciliary body, sclera, choroid, and retina were dissected, collected, weighed and stored at − 70 °C until assay. One set of instruments was used per eye.
Drug concentrations were measured by employment of standard HPLC-TMS (“high-performance liquid chromatography–tandem mass spectrometry”) method by an independent lab (Intertek, San Diego, CA). Validated methodology was used to produce precision, stability, and accuracy. These procedures were performed within rigorous standards (described below). All institutional and national guidelines for the care and use of laboratory animals were followed.
Bromfenac and an internal standard (Amfenac) were extracted from aqueous humor by protein precipitation using acetonitrile. The supernatants were evaporated to dryness and reconstituted in 50:50 ACN:0.1% FA. The extracts were analyzed by liquid chromatography–mass spectrometry (LC–MS/MS) using an API5000TM mass spectrometer (AB Sciex, Ontario, Canada).
Ocular tissue methods used Tolmetin for the internal standard. Ocular tissues were homogenized in 100 mM NH4HCO3, pH 10 buffer using an Omni Bead Ruptor 24 with ceramic beads. Another cycle of homogenization was run after addition of 90:10 ACN:DMSO. For sclera and ICB, a portion of the homogenate was extracted with ethyl acetate after addition of 1 M NH4OAc buffer pH 4. The organic layer was evaporated to dryness and reconstituted in 50:50 ACN:0.1% FA. For choroid and retina, a portion of the homogenate was processed by solid phase extraction using Strata X, 30 mg cartridges (Phenomenex, Torrance, CA).
After cartridge conditioning, samples were loaded, the cartridges rinsed with 10:90 MeOH:H2O and the analytes eluted with methanol. The eluate was evaporated to dryness and reconstituted in 50:50 ACN:0.1% FA. All tissue extracts were analyzed by LC–MS/MS using an API4000TM mass spectrometer (AB Sciex, Ontario, Canada).
For chromatography, an Ace Excel 2 C18-AR, 50 × 2.1 mm, 2 µm column (Advanced Chromatography Technologies Ltd, Aberdeen, Scotland) was used with a gradient method having mobile phase A (0.1% formic acid) and mobile phase B (0.1% formic acid in acetonitrile). The following transitions were monitored: m/z 334.2 → 288.1 (Bromfenac), m/z 256.2 → 210.2 (Amfenac) or m/z 258.2 → 119.1 (Tolmetin). Methods were linear using 1/× 2 weighting. The assay range for aqueous humor was 0.5–250 ng/m,L and for the ocular tissues was 0.1–100 ng (concentrations in tissue samples were corrected for collected weight after analysis).
Pharmacokinetic (PK) parameters including mean tissue concentration and its 95% confidence interval, maximum concentration (Cmax) and time to maximum concentration (Tmax), area under the curve (AUC0.5–24h) were reported. Mean Cmax was estimated by geometric means based on log-transferred data and the geometric means ratio was used to assess the differences between treatment groups. AUC was estimated using a linear trapezoidal method for sparse sampling. To test the gender effect and the gender-by-treatment interaction effect, a saturated linear mixed effects model was fitted first and the effects were found to not be statistically significant. Therefore, the gender effect was dropped from the rest of the analysis. Statistical analyses were performed using SAS (Version 9.4, SAS Institute, Cary, NC). The significance level was set to p < 0.05 for all tests. ANOVA test was used for between-group comparisons.
Results
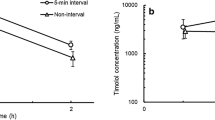
Similar ocular distribution patterns were seen in both the anterior and posterior segments. NSAID concentration results are presented by matrix, from anterior to posterior (Fig. 1a–e). Amfenac concentrations are reported for Ilevro®. Representative concentration-over-time graphs are provided for the anterior (aqueous humor, iris-ciliary body, sclera) and posterior segment (choroid, retina) matrices.
Aqueous Humor Analyte Pharmacokinetics
The NSAID aqueous humor Cmax of BromSite® BID was 1.7-, 2.2- and 3.7-fold higher than that of BromSite® QD, Prolensa®, and Ilevro®, respectively. The Cmax for Ilevro® occurred at 1 h and for the other three groups at 2 h (Fig. 1a). The Bromsite® BID group had the highest Cmax at 131 µg/mL, followed by Bromsite® QD (77 µg/mL) and Prolensa® (59 µg/mL), while Ilevro® group was much lower at 35 µg/mL. The Cmax in the Bromsite® BID group was statistically significantly higher (p < 0.05) than that of Prolensa® and Ilevro®.
The NSAID aqueous humor AUC concentration of bromfenac from BromSite® BID was 1.7-, 1.8, and 3.6-fold higher compared to the NSAID concentrations in BromSite® QD, Prolensa®, and Ilevro®, respectively. The differences in AUC were statistically significant (p < 0.05) between BromSite® BID and the other three groups.
Iris-Ciliary Body Analyte Pharmacokinetics
The NSAID iris-ciliary body Cmax occurred at 0.5 h for BromSite® BID and at 1 h for the other three groups (Fig. 1b). The Cmax of BromSite® BID was 1.6-, 3.0-, and 4.3-fold that of BromSite® QD, Prolensa®, and Ilevro®, respectively. The Cmax in the Bromsite® BID group was statistically significantly higher (p < 0.05) than that of Ilevro®.
The iris-ciliary body AUC concentration of bromfenac from BromSite® BID was 1.5-, 1.9-, and 2.9-fold higher compared to the NSAID concentrations in BromSite® QD, Prolensa®, and Ilevro®, respectively. The differences in Cmax and AUC between BromSite® BID and the other three groups were statistically significant (p < 0.05).
Sclera Analyte Pharmacokinetics
The NSAID scleral Cmax occurred at 0.5 h for BromSite® QD and Ilevro®, and at 1 h for BromSite® BID and Prolensa® (Fig. 1c). The Cmax of BromSite® BID was similar to BromSite® QD (geometric means ratio = 1.04), but 2.7-, and 2.5-fold greater than that of Prolensa®, and Ilevro®, respectively. The differences between BromSite® BID and Prolensa®, as well as between BromSite® BID and Ilevro® were statistically significant (p < 0.05).
The scleral AUC concentration of bromfenac from BromSite® BID was approximately the same compared to BromSite® QD (AUC ratio = 1.03) and was 1.8- and 3.5-fold higher compared to the NSAID concentrations in Prolensa® and Ilevro®, respectively. The differences in AUC were statistically significant (p < 0.05) between BromSite® BID and Prolensa®, as well as between BromSite® BID and Ilevro®.
Choroid Analyte Pharmacokinetics
The NSAID Choroid Cmax occurred at 0.5 h for BromSite® QD and Ilevro®, and at 1 h for BromSite® BID and Prolensa® (Fig. 1d). The Cmax of BromSite® BID was 1.6-, 3.0-, and 4.2-fold higher than that of BromSite® QD, Prolensa®, and Ilevro®, respectively. Statistically significant (p < 0.05) differences were detected between BromSite® BID and Prolensa®, and between BromSite® BID and Ilevro®.
The choroidal AUC concentration of bromfenac from BromSite® BID was 1.5-, 2.5- and 3.0-fold higher compared to the NSAID concentrations in BromSite® QD, Prolensa®, and Ilevro®, respectively. The AUC in the BromSite® BID group was statistically significantly higher than the other three groups (p < 0.05).
Retina Analyte Pharmacokinetics
The NSAID retinal Cmax occurred at 0.5 h for BromSite® BID and Prolensa®, and at 1 h for BromSite® QD and Ilevro® (Fig. 1e). The Cmax of BromSite® BID was 2.1-, 7.7-, and 8.6-fold higher than that of BromSite® QD, Prolensa®, and Ilevro®, respectively. The Cmax of BromSite® BID was statistically significantly higher than that of Ilevro® (p < 0.05).
The retinal AUC concentration of bromfenac from BromSite® BID was 1.5-, 2.8-, and 2.3-fold higher compared to the NSAID concentrations in BromSite® QD, Prolensa®, and Ilevro®, respectively. The differences in AUC was statistically significant between BromSite® BID and the other three groups (p < 0.05). The concentration over time graph (Fig. 1e) indicates BromSite® BID provides sustained concentrations over time in the retina.
Discussion
This study provided some key scientific data. Although the concentration patterns were highly consistent, human tissue distribution does not necessarily correlate with rabbit tissue concentrations. Furthermore, this experiment did not include cataract surgery, so post-operative alterations in blood flow and tissue inflammation could also selectively alter concomitant NSAID tissue concentrations.
This study utilized 6 rabbits per group, so tighter confidence intervals with larger cohorts might better support the conclusions. From a methodological and statistical perspective for mass as small as retina or a tissue as dense as the sclera, for instance, future studies could evaluate more animals per time point. While our data are valid, we believe the two- to threefold higher concentrations we reported for BromSite® would have reached statistical significance more frequently with larger study groups. This can especially be appreciated when reviewing the Cmax results compared to the AUC results: AUC results were generally more robust throughout, with far more statistically significant end point differences.
Walters and colleagues previously reported PK results of a single-dose human aqueous humor study comparing amfenac, ketorolac, and bromfenac [15]. Their study was published in 2007 and evaluated the relevant comparators (i.e., Nevanac®, Acular LS® and Xibrom®) at that time. The current study herein is a contemporary assessment of the relevant comparators for 2017 (i.e., BromSite®, Prolensa® and Ilevro®) in both the anterior and posterior segments within an animal model.
Intraocular NSAID concentrations are expected to correlate with the efficacy of a given drug. While aqueous humor is an important matrix to consider, NSAIDs are prescribed, albeit off-label, for posterior segment efficacy as well. This study provides the first PK understanding of currently used products with anterior segment administration and posterior segment therapeutic implications.
While the concentration of NSAIDs in the various tissues is important, Cyclooxygenase-1 (COX-1) and Cyclooxygenase-2 (COX-2) enzymatic activity and the inhibition of both enzymes is foundational to understanding the pharmacologic actions of each NSAID [15]. COX-1 is an omnipresent protein important to homeostatic activity, such as maintenance of renal function, platelet aggregation, and gastric protection. Comparatively, COX-2 is an enzyme that is induced and largely responsible for prostaglandin creation during trauma within various tissues [16], and has been well-researched in models of ocular trauma as an enzyme responsible for increased prostaglandin activity [17,18,19,20].
In the Walters study, there was an examination of cyclooxygenase inhibition pharmacodynamic (“PD”) results. The results of these studies are independent of product and entirely dependent on methodology. For COX-1 sheep inhibitory activity, the authors reported ketorolac as the most potent. In the case of human recombinant COX-2 assay, the most potent was reported as amfenac. In these same models the authors stated COX-2 and COX-1 inhibition was intermediary for bromfenac.
A more recent PK/PD study was reported by Kida et al. [10]. This study evaluated diclofenac, amfenac and bromfenac and found a rank order of potency at human platelet COX-1 and human recombinant COX-2 to be the same for each cyclooxygenase isoform: bromfenac > amfenac > diclofenac (ranked most to least potent). As pointed out by Walters et al. [15] interpretation of results across studies is challenging due to differing methodologies, instrumentation, etc. Thus, small changes in these assays may lead to differing results. As stated by Walters and colleagues, “because many NSAIDs are time-dependent inhibitors, increasing assay incubation times will result in lower (more potent) IC50 values. Likewise, variations in other assay conditions (e.g., temperature, source of enzymes, measuring oxygen consumption versus PG production) will affect the results”. Walters and Kida both measured isoforms under the same temperature (37 °C) but different times of incubation (COX-1/COX-2): 2/2 min vs. 15/5 min for Walters and Kida, respectively.
Kida and colleagues used products commercially available, at the time, for the in vivo/PK portion of their study [10]. It should be noted the nepafenac was the 0.1% solution while the bromfenac was the 0.09% ophthalmic solution. The Cmax of diclofenac, bromfenac, and amfenac in the retinochoroidal tissue were 38.2 ng/g at 1 h, 15.8 ng/g at 30 min, and 12.5 ng/g at 30 min, respectively. The drug clearance of diclofenac was rapid, with an elimination half-life (T1/2) of 1.89 h, whereas the clearances of bromfenac and amfenac were relatively slow with the T1/2 at 4.69 and 4.00 h, respectively. Modeled PK in AH and retinochoroidal tissues demonstrated levels of the 3 NSAIDs were constantly higher than the IC50 of COX-2 in the AH, however, only bromfenac was constantly greater than the IC50 at its trough level in the retinochoroidal tissues.
There were no studies found in the literature comparing single-dose AUC to multiple-dose AUC for NSAIDs. However, Si and colleagues examined aqueous concentrations for both dosing regimens and found a 1.8-fold higher concentration at 2 h for the multiple-dose (i.e., three times daily for 14 days) DuraSite® 0.09% formulation compared to the single-dose DuraSite® 0.09% formulation. The increased concentration was also seen in the commercial 0.09% formulation. That is, there was a 1.4-fold higher accumulation with the multiple-dose vs. single-dose. Thus, the Si study suggests there to be accumulation of bromfenac with multiple-dose regimens compared to single-dose regimens, regardless of the formulation. Furthermore, this difference is more pronounced with the DuraSite® vehicle.
Si also demonstrated that the tissue concentration of bromfenac in DuraSite® was significantly higher than that of Xibrom® everywhere excluding the retina. The bromfenac concentration in the choroidal tissue achieved by the DuraSite® formulation, in particular, was approximately three times higher than that obtained by Xibrom®. In the current study, when BromSite® Cmax data were compared to Prolensa® data, sevenfold higher retina concentrations were noted, despite very similar NSAID formulation concentrations. BromSite® contains 0.075% bromfenac and Prolensa® ophthalmic solution contains 0.07% bromfenac. Thus, the current study, unlike Si et al. found that DuraSite® provides remarkably higher retinal bioavailability of bromfenac.
It is anticipated that higher Cmax and AUC values will translate to improved efficacy of any NSAID in inhibiting the synthesis of PGs, resulting in a quicker resolution of inflammation and pain following cataract surgery, as well as prevention or reduction of the incidence of CME [21]. Posterior segment efficacy of the bromfenac molecule has been well documented in human clinical literature, [22] offering promise to surgeons and their patients.
There are three noteworthy limitations to the current study. First, there is a large standard deviation in the ocular matrices concentrations. To address these limitations, we suggest undertaking a study with a larger sample size in the future; this would presumably allow significant differences where there are currently large numerical differences. Secondly, an animal model was used for understanding ocular distribution in the human. This second limitation is not easily overcome, unless in another animal species. Thirdly, topical ocular dosing was tightly controlled and may not be reflective of human compliance to topical ocular NSAID dosing.
Conclusions
DuraSite® significantly enhances the ocular bioavailability of bromfenac in BromSite®, thus, contributing to significantly higher NSAID concentrations throughout both anterior and posterior segment ocular tissues compared to Prolensa® or Ilevro®.
Change history
19 February 2019
In the original publication, units were incorrectly published in the results section as micrograms (µg/mL).
References
Ahuja M, Dhake AS, Sharma SK, Majumdar DK. Topical ocular delivery of NSAIDs. AAPS J. 2008;10:229–41. https://doi.org/10.1208/s12248-008-9024-9.
Colin J. The role of NSAIDs in the management of postoperative ophthalmic inflammation. Drugs. 2007;67:1291–308. https://doi.org/10.2165/00003495-200767090-00004.
Schalnus R. Topical nonsteroidal anti-inflammatory therapy in ophthalmology. Ophthalmologica. 2003;217:89–98. https://doi.org/10.1159/000068563.
Abel R, Abel AD. Perioperative antibiotic, steroidal, and nonsteroidal anti-inflammatory agents in cataract intraocular lens surgery. Curr Opin Ophthalmol. 1996;7:39–42. https://doi.org/10.1097/00055735-199708010-00007.
Polansky J, Weinreb R. Steroids as anti-inflammatory agent. In: Sears M, editor. Pharmacology of the eye. New York: Springer; 1984. p. 460–583.
Raizman M. Corticosteroid therapy of eye diseases: fifty years later. Arch Ophthalmol. 1996;114:1000–1.
Jones BM, Neville MW. Nepafenac: an ophthalmic nonsteroidal anti-inflammatory drug for pain after cataract surgery. Ann Pharmacother. 2013;47:892–6.
Kim SJ, Schoenberger SD, Thome JE, Ehlers JP, Yeh S, Bakri SJ. Topical nonsteroidal anti-inflammatory drugs and cataract surgery, a report by the american academy of ophthalmology. Ophthamology. 2015;122:2159–68.
Walsh DA, Moran HW, Shamblee DA, Uwaydah IM, Welstead WJ, et al. Anti-inflammatory agents. 3. Synthesis and pharmacological evaluation of 2-amino-3-benzoylphenyl acetic acid and analogues. J Med Chem. 1984;11:1379–88.
Kida T, et al. Pharmacokinetics and efficacy of topically applied nonsteroidal anti-inflammatory drugs in retinochoroidal tissues in rabbits. PLoS One. 2014;9(5):e96481.
Baklayan GA, Patterson HM, Song CK, Gow JA, McNamara TR. 24-Hour evaluation of the ocular distribution of 14C-labeled bromfenac following topical instillation into the eyes of New Zealand white rabbits. J Ocul Pharmacol Ther. 2008;24(4):392–8.
Si EC, Bowman LM, Hosseini K. Pharmacokinetic comparisons of bromfenac in durasite and xibrom. J Ocul Pharmacol Ther. 2011;27(1):61–6.
Bromsite™ United States Prescribing Information. Revised 04/2016.
Si EC, Bowman LM, Hosseini K. Pharmacokinetic comparisons of bromfenac in durasite and xibrom. J Ocul Pharmacol Ther. 2010;27(1):61–6.
Walters T, Raizman M, Ernest E, Gayton J, Lehman J. In vivo pharmacokinetics and in vitro pharmacodynamics of nepafenac, amfenac, ketorolac, and bromfenac. J Cataract Refract Surg. 2007;33:1539–45.
Dubois RN, Abramson SB, Crofford L, et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–73.
Oka T, Shearer TR, Azuma M. Involvement of cyclooxygenase-2 in rat models of conjunctivitis. Curr Eye Res. 2004;29:27–34.
Guex-Crosier Y. Anti-inflammatoires non stéroïdiens (AINS) et inflammation oculaire [Non-steroidal anti-inflammatory drugs and ocular inflammation.]. Klin Monatsbl Augenheilkd. 2001;218:305–8.
Bonazzi A, Mastyugin V, Mieyal PA, et al. Regulation of cyclooxygenase-2 by hypoxia and peroxisome proliferators in the corneal epithelium. J Biol Chem. 2000;275:2837–44.
Masferrer JL, Kulkarni PS. Cyclooxygenase-2 inhibitors: a new approach to the therapy of ocular inflammation. Surv Ophthalmol. 1997;41(suppl 2):S35–40.
Shafiee A, Bowman LM, Hou E, Hosseini K. Aqueous humor penetration of ketorolac formulated in durasite or durasite 2 delivery systems compared to acular LS in rabbits. J Ocul Pharmacol Ther. 2013;29(9):812–6.
Sheppard JD. Topical bromfenac for prevention and treatment of cystoid macular edema following cataract surgery: a review. Clin Ophthalmol. 2016;10:2099–111.
Acknowledgements
Funding
This study and publication processing costs were supported by Sun Pharmaceutical Industries Ltd. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing and Editorial Assistance
The authors would like to acknowledge the contributions of Sinclair Research (in vivo) and Intertek (bioanalytical) portions of this study; Jenny Song, MD, MS for the analyses, and Paul C. Cockrum for project management and medical writing.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
John D. Sheppard: Commercial Relationship(s): Virginia Eye Consultants. (Consultant). Paul C. Cockrum: Commercial Relationship(s); HealthKinetics: Contractor (Consultant). Angela Justice: Commercial Relationship(s): Sun Pharmaceutical Industries, Inc. (Employment). Mark C. Jasek: Commercial Relationship(s): Sun Pharmaceuticals Industries, Inc. (Employment).
Compliance with Ethics Guidelines
All institutional and national guidelines for the care and use of laboratory animals were followed.
Data Availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.6126956.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Sheppard, J.D., Cockrum, P.C., Justice, A. et al. In Vivo Pharmacokinetics of Bromfenac Ophthalmic Solution 0.075%, Bromfenac Ophthalmic Solution 0.07%, and Nepafenac/Amfenac Ophthalmic Suspension 0.3% in Rabbits. Ophthalmol Ther 7, 157–165 (2018). https://doi.org/10.1007/s40123-018-0130-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-018-0130-1