Abstract
Introduction
Povidone iodine (PVP-I) 10% aqueous solution is a commonly utilized anti-septic employed for sterilization of the ocular surface prior to interventional procedures. Dimethylsulfoxide (DMSO) is a well-known skin penetration agent scarcely utilized in ophthalmic drug formulations. We describe here a low-dose formulation of 1% PVP-I (w/w) in a gel containing DMSO for use in the setting of recalcitrant rosacea blepharoconjunctivitis. A review of the ocular uses of dimethylsulfoxide is also presented.
Case report
A 78-year-old male presented with chronic, long-standing blepharitis involving both the anterior and posterior lid margins. Posterior lid and skin inflammatory changes were consistent with ocular rosacea. Previous oral and topical therapies had been largely ineffective at controlling his condition.
Conclusion
The topical PVP-I/DMSO system was effective in abating the signs and symptoms of rosacea blepharoconjunctivitis. Further investigation of this novel agent is warranted.
Similar content being viewed by others
Introduction
Blepharitis is a common ocular condition presenting in a large percentage of the population [1, 2]. For most sufferers profound visual impairment is rare, however, a majority will demonstrate chronic ocular surface dysfunction including evaporative dry eye [3]. There are a variety of causal factors implicated in blepharitis including bacterial overgrowth, yeast colonization, viral infection, Demodex mites, atopy, seborrhea, environmental factors, hormonal dysregulation, and rosacea [4]. Rosacea is a chronic inflammatory skin condition with multiple, distinct subtypes that affects approximately 16 million Americans [5]. Transient and nontransient facial flushing, telangiectasia, and inflammatory papules and pustules represent the most commonly recognized skin manifestations [5].
To more accurately diagnose and treat blepharitis, the disease is frequently subdivided based on anatomical position. Anterior blepharitis is frequently associated with Gram-positive bacterial overgrowth or seborrhea [6]. Common findings include eyelid erythema, scurf, collarettes, lid margin thickening or tylosis ciliaris, and breakage or misdirection of cilia. Posterior blepharitis most commonly manifests as meibomian gland dysfunction with other findings that may include lid erythema, telangiectatic lid margin vasculature, hyperkeratosis, chalazia, and tarsal inflammation.
It is largely recognized that a spectrum of meibomian gland dysfunction exists in those patients suffering from chronic posterior blepharitis [7]. Each of these holocrine glands consists of multiple acini connected to a long central duct, which secretes lipids comprised mainly of wax and sterol esters. Under the effect of chronic inflammation, they may develop pouting, dropout, dilation, and epithelial hyperkeratinization. Furthermore, changes in the viscosity of lipid secretions due to structural shifts of saturated hydrocarbons and protein interaction may contribute to deranged tear homeostasis [7, 8].
Microorganisms likely play a crucial role in the development of blepharitis. Normal ocular surface colonizers include Gram-positive bacteria, especially coagulase-negative Staph species (CoNS), Streptococcus, Corynebacterium, and Propionibacterium acnes [9]. In the setting of anterior blepharitis, CoNS are known to release toxic by-products thus triggering an inflammatory cascade. Acting upon the posterior lid, some bacterial species are imbued with lipases and esterases which cleave meibum into free fatty acids and soaps. Moreover, loss of polar lipids located between nonpolar and aqueous components of the tear film may also decrease lipid spreading. All these actions serve to propagate further inflammation and destabilize the tear film. Bacteria not only colonize the superficial ocular surface, but evidence suggests that they may be present within deeper structures like the meibomian orifices and ducts [10]. The pathophysiological process has not been fully elucidated; however, it can be postulated that deep intraductal bacteria may contribute to meibum alterations by acting upon them prior to formation of the mature secretion.
Given the varied manifestations and treatment recalcitrance of blepharitis, it is not surprising that though many formulations have been tried, they have demonstrated only modest benefit. None are currently approved by the food and drug administration (FDA) for this indication.
Case Report
Informed consent was obtained from the patient for publication of this case report. A 78-year-old male with a past ocular history of glaucoma and pseudophakia presented with long-standing ocular dryness, grittiness, periocular erythema, and eyelid crusting. On facial inspection, nasal and facial telangiectasias with flushing were evident. His topical medical regimen included one drop of latanoprost at night in both eyes, one drop of brimonidine in both eyes twice daily, and one drop of dorzolamide/timolol in both eyes twice daily. The patient endorsed utilization of a variety of medicines and treatments to alleviate this condition; however, they were of little benefit. Failed therapies included topical prednisolone acetate, loteprednol, azithromycin, cyclosporine, and combination medicines such as tobramycin/dexamethasone and neomycin/polymyxin B/dexamethasone. Oral therapies including doxycycline, and fish and flax seed oils were also ineffective.
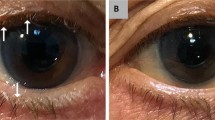
Slit lamp biomicroscopic examination revealed bilateral anterior lid margin erythema, crusting, and thickening. Lash examination revealed some breakage with scurf-like deposition. Inspection of the posterior eyelids revealed inspissated meibomian glands with capping and turbid secretions. Further towards the posterior tarsal area, dilated, engorged telangiectatic vessels were present. The marginal lid erythema extended not only to the tarsal plate, but also to the inferior bulbar conjunctiva. Tear break-up time was notably decreased and corneae revealed inferior, bilateral punctate epithelial erosions. A diagnosis of rosacea blepharoconjunctivitis was made.
The patient was given a topical gel of 1% PVP-I in a dimethylsulfoxide (DMSO) vehicle that was prepared by a licensed compounding pharmacy. The treatment was administered twice daily and delivered by rubbing the gel onto the lash line and eyelid. At the first follow-up visit one week later, remarkable improvements were noted. Most prominently, much of the conjunctivitis, anterior lid erythema, and thickening had reversed. The patient was instructed to decrease the gel to once daily, but to continue treatment for a total of one month. At this second follow-up visit, not only were the initial improvements conserved, but the posterior lid margin vessels and telangiectasias had begun to attenuate and involute. Moreover, meibomian capping was no longer present; secretions were less viscous and tear break-up time normalized. Besides occasional mild tingling at the application site, the patient reported no other adverse effects.
Discussion
Blepharitis is often a recalcitrant, chronic ocular condition with variable presentation. Fortunately, sight-threatening manifestations of this disease such as lid cicatrization, malposition, trichiasis, and corneal infiltrates are rare. Nonetheless, morbidity imparted in the form of chalazia, rosacea, dry eye, contact lens intolerance, and even anxiety is significant [11, 12]. The complex nature of blepharitis renders curative treatment challenging as there are currently no FDA-approved treatments. It has been postulated that because of the inflammatory and infectious natures of the disease that controlling both of these variables may form the bedrock for effective therapy [13].
PVP-I has been recognized as a safe, effective, broad-spectrum, biocidal agent for many years [14]. It is primarily utilized in ophthalmology for antisepsis prior to procedures that place patients at risk for endophthalmitis [14]. There are a variety of other indications within ophthalmology where low-dose (i.e., less than 10% w/w) PVP-I formulations have been shown to be useful [15, 16]. Though incompletely understood, it is likely its mechanism of action relates to free iodide which poisons electron transport, inhibits cellular respiration, and destabilizes membranes. Ocular use of PVP-I has been highlighted previously by the authors [17].
DMSO is an organic, polar, aprotic molecule utilized as a pharmaceutical solvent for decades [18]. The term aprotic refers to the lack of H+ ion available for proton donation in Brønsted acid reactions. DMSO was first synthesized by Alexander Saytzoff, a Russian chemist in 1867. After remaining shrouded in obscurity for almost a century, it was rediscovered in the 1950s for its ability to act as a “super solvent”. Notably, the DMSO chemical structure yields a polar solvent with a high dielectric constant. This property along with favorable stereochemistry is thought to contribute to its prodigious solvating ability [19].
DMSO is commonly produced as a by-product of paper manufacture from lignin, a fiber found in wood pulp. Its early utility focused upon organ preservation studies because of an unusual, dramatic ability to depress the freezing point of water while maintaining cellular viability [20]. The development of DMSO as an active pharmaceutical agent was halted in the late 1960s due to a perceived lack of “patentability” rendering commercialization a challenge. The general lack of funding in conjunction with increased vigilance by the FDA in light of the thalidomide scares brought the development of DMSO to a standstill. In 1978, the FDA finally approved a 50% DMSO solution for intravesicular delivery to treat interstitial cystitis (RIMSO-50, NDA#017788, Mylan). Since 1978, DMSO has been used in a variety of FDA-approved drug products as an inactive solvent and most recently in a topical diclofenac formulation for knee-pain, Pennsaid® (NDA #020947, Nuvo Research Inc.).
The clinical utility of DMSO seems to derive from two broad, imperfectly understood mechanistic classes—radical scavenging and penetration enhancement. DMSO is considered a free radical trap. It is postulated that free radical scavenging is responsible for the abundant anecdotal reports of DMSO as a cure for many skin, eye, and arthritic conditions. Radical scavenging in general can be viewed as an “anti- inflammatory” process and many of the claims for DMSO utility are based on some variation of suppressing the inflammatory response [21].
There are four variables that influence penetration of a solute through any given membrane: (1) the diffusion coefficient through the membrane, (2) the concentration of the agent in the vehicle, (3) the partition coefficient between the membrane and the vehicle, and (4) the thickness of the membrane barrier. Penetration agents are designed to affect one or more of these variables without causing permanent structural or chemical modification of the physiological barrier. DMSO is very well characterized as a membrane penetration enhancement agent [22]. The mechanism primarily involves alteration of the diffusion and partition coefficients at the membrane level. These properties are due in part to favorable reactions with membrane lipid head groups and the production of more permeable packing arrangements. DMSO is also considered a small amphiphile and has a propensity for rapid incorporation at the membrane lipid–water interface. Studies have highlighted the ability of DMSO to induce water pores within lipid bilayers, thereby forming a conduit for active molecules to enter cells [23]. As previously discussed, DMSO can also have a profound effect on aqueous solubility of less soluble agents and has been employed in a variety of pharmaceutical preparations. These interactions remain subject to molecular weight, shape, and chemistry afforded by the solvent; however, the end result is the delivery of a greater concentration of solute to the target.
It is generally considered that ocular DMSO utilization is so ubiquitous that it remains vastly underreported. The non-peer-reviewed literature and the Internet in general are rife with anecdotal reports praising its utility and safety in blepharitis, cataracts and for the treatment of certain retinal diseases. Fortunately, there also exists a robust body of peer-reviewed literature that forms the bedrock for its safe use in humans. In 1968, Gordon published a seminal work on extended utilization of DMSO in the human eye. He reported on 157 eyes in which DMSO (7.5–66%) was employed for various ophthalmic indications [24]. These patients were followed for a period of up to 19 months without the observation of any ocular toxicity. At that time, these were vital findings to the future of the solvent as early animal testing had demonstrated curious lenticular findings in canines [24]. These findings were not necessarily cataractous in nature, but represented changes in translucency between the lens nucleus and cortex along with myopic shift. Other studies performed on monkeys, guinea pigs, rabbits and later with cell culture corroborated these observations [25, 26].In a more encompassing report on human utilization from the Second International DMSO Symposium in Vienna, an astounding 9521 patients were followed and treated with DMSO for up to 2.5 years [27]. There was not a single instance of lens toxicity reported in any of these study participants. In 1973, a landmark report published in the Annals New York Academy of Sciences further supported its safe human use. Here, a topical DMSO aqueous solution was administered daily to 65 patients with up to 4–7 years of follow-up. No observable toxicity was found outside of transient irritation and occasional conjunctival erythema [28]. More recently, one case report of DMSO-related lenticular pigmentary change was noted in a woman being treated for interstitial cystitis [29]. Her treatment was with RIMSO-50, administered via multiple bladder washouts. The pigmentary changes were thought to be responsible for mild hypermetropic shift; however, no change in best corrected visual acuity was noted.
Other animal studies with DMSO have also been performed yielding notable results. In 1977, a concentration-dependent exacerbation of conjunctival inflammation was observed for DMSO concentrations >90% (w/w, aqueous). The authors found that 90% and 100% DMSO worsened ocular inflammation, while doses lower than 30% demonstrated anti-inflammatory properties [30]. Other studies conducted in 2010 and 2011 saw its administration both subconjunctivally and following trabeculotomy [31, 32]. A synopsis of published DMSO ocular findings is detailed in Table 1.
Our current understanding with respect to preferential distribution of DMSO in the eye is that uptake is most consistently found in the cornea, aqueous, vitreous and sclera [33, 34]. Much of this data stems from sacrificial animal studies performed in the 1960s. More recently, a vitrification study for cryoprotective purposes reconfirmed corneal uptake [35]. It is also interesting to note that although animal studies have endorsed lenticular alterations, to our knowledge, DMSO does not accumulate in the crystalline lens.
With respect to our patient in this case report, it is not surprising that there appears to be a demonstrable response in the setting of rosacea blepharoconjunctivitis. The eyelid structures represent therapeutic targets with architecture that is distinct in anatomy and function [36]. Notably, it is upon the lid margin skin where many of the microorganisms responsible for causing devastating post-surgical ocular infections reside [14]. Lid margin bacteria not only populate and thrive on the superficial lid tissues but may ensconce within the deep eyelid or within dead layers of skin cells, sweat glands, and hair follicles [37]. It is logical, therefore, that successful therapy should fundamentally offer both antisepsis and lid penetration with significant anti-inflammatory effect. This is the postulated mechanism of action the authors endorse with respect to the novel formulation discussed in this manuscript.
It is important to note the shortcomings of this case report. There was no utilization of a blepharitis or eyelid vasculature/erythema scoring scale, high-resolution external photographs were not submitted, and there was no scoring of corneal damage. Lastly, no bacterial cultures of the lid margin or conjunctival sac were taken to confirm anti-septic effect.
Conclusions
Both DMSO and PVP-I have been known separately in the medical and pharmaceutical literature for decades. No one had previously contemplated their combined use in ophthalmic formulations until the current report. We have shown possible success with this novel formulation of low-dose PVP-I in a proprietary DMSO gel/solvent system for a difficult case of rosacea blepharoconjunctivitis. This novel therapy may warrant further investigation in randomized, controlled clinical trials.
References
Lemp MA, Nichols KK. Blepharitis in the United States 2009: a survey-based perspective on prevalence and treatment. Ocul Surf. 2009;7(2 Suppl):S1–14.
Pflugfelder SC, Karpecki PM, Perez VL. Treatment of blepharitis: recent clinical trials. Ocul Surf. 2014;12(4):273–84.
Mathers W. cluster analysis of patients with ocular surface disease, blepharitis, and dry eye. Arch Ophthalmol. 122(11):1700–4.
American Academy of Ophthalmology. Preferred practice patterns: blepharitis. 2008. Available from: http://one.aao.org/CE/PracticeGuide-lines/PPP.aspx.
Two AM, Wu W, Gallo RL, Hata TR. Rosacea: part I. Introduction, categorization, histology, pathogenesis, and risk factors. J Am Acad Dermatol. 2015;72(5):749–58.
Duncan K, Jeng BH. Medical management of blepharitis. Curr Opin Ophthalmol. 2015;26(4):289–94.
Mcculley JP, Shine WE. Meibomian gland function and the tear lipid layer. Ocul Surf. 2003;1(3):97–106.
Oshima Y, Sato H, Zaghloul A, Foulks GN, Yappert MC, Borchman D. Characterization of human meibum lipid using raman spectroscopy. Curr Eye Res. 2009;34(10):824–35.
O’brien TP. The role of bacteria in blepharitis. Ocul Surf. 2009;7(2 Suppl):S21–2.
Dougherty JM, Mcculley JP. Comparative bacteriology of chronic blepharitis. Br J Ophthalmol. 1984;68(8):524–8.
Nemet AY, Vinker S, Kaiserman I. Associated morbidity of blepharitis. Ophthalmology. 2011;118(6):1062–8.
Chiang CC, Lin CL, Tsai YY, Peng CL, Liao YT, Sung FC. Patients with blepharitis are at elevated risk of anxiety and depression. PLoS One. 2013;8(12):e83335.
Luchs J. Azithromycin in DuraSite for the treatment of blepharitis. Clin Ophthalmol. 2010;4:681–8.
Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology. 1991;98(12):1769–75.
Pelletier JS, Stewart K, Trattler W, et al. A combination povidone-iodine 0.4%/dexamethasone 0.1% ophthalmic suspension in the treatment of adenoviral conjunctivitis. Adv Ther. 2009;26(8):776–83.
Isenberg SJ, Apt L, Valenton M, et al. A controlled trial of povidone-iodine to treat infectious conjunctivitis in children. Am J Ophthalmol. 2002;134(5):681–8.
Abelson M, Capriotti JA, Shapiro A, Lilyestrom L. Iodine: an elemental force against infection. Rev Ophthalmol. 2009;15(6):36–42.
Jacob SW, De la torre JC. Pharmacology of dimethyl sulfoxide in cardiac and CNS damage. Pharmacol Rep. 2009;61(2):225–35.
Srivastava KR, Kumar A, Goyal B, Durani S. Stereochemistry and solvent role in protein folding: nuclear magnetic resonance and molecular dynamics studies of poly-L and alternating-L, D homopolypeptides in dimethyl sulfoxide. J Phys Chem B. 2011;115(20):6700–8.
Akkok CA, Liseth K, Hervig T, et al. Use of different DMSO concentrations for cryopreservation of autologous peripheral blood stem cell grafts does not have any major impact on levels of leukocyte- and platelet-derived soluble mediators. Cytotherapy. 2009;11(6):749–60.
Hanna C, Fraunfelder FT, Meyer SM. Effects of dimethyl sulfoxide on ocular inflammation. Ann Ophthalmol. 1977;9(1):61–5.
Stoughton RB, Fritsch W. Influence of dimethylsulfoxide (DMSO) on human percutaneous absorption. Arch Dermatol. 1964;90:512–7.
Notman R, Den otter WK, Noro MG, Briels WJ, Anwar J. The permeability enhancing mechanism of DMSO in ceramide bilayers simulated by molecular dynamics. Biophys J. 2007;93(6):2056–68.
Gordon DM, Kleberger KE. The effect of dimethyl sulfoxide (DMSO) on animal and human eyes. Arch Ophthalmol. 1968;79(4):423–7.
Barnett KC, Noel PRB. Dimethyl sulfoxide and lens changes in primates. Nature. 1967;214:1115.
Cao XG, Li XX, Bao YZ, Xing NZ, Chen Y. Responses of human lens epithelial cells to quercetin and DMSO. Invest Ophthalmol Vis Sci. 2007;48(8):3714–8.
Laudahn G, Gertich K. Dimethyl-Sulfoxyd Internationale Symposium, Berlin; 1966.
Hill RV. Dimethyl sulfoxide in the treatment of retinal disease. Ann NY Acad Sci. 1975;243:485–93.
Rowley S, Baer R. Lens deposits associated with RIMSO-50 (dimethylsulphoxide). Eye (Lond). 2001;15(Pt 3):332–3.
Galvao J, Davis B, Tilley M, Normando E, Duchen MR, Cordeiro MF. Unexpected low-dose toxicity of the universal solvent DMSO. FASEB J. 2014;28(3):1317–30.
Piña Y, Decatur C, Murray T, et al. Advanced retinoblastoma treatment: targeting hypoxia by inhibition of the mammalian target of rapamycin (mTOR) in LH(BETA)T(AG) retinal tumors. Clin Ophthalmol. 2011;5:337–43.
Lüke J, Nassar K, Lüke M, et al. The effect of adjuvant dimethylenastron, a mitotic Kinesin Eg5 inhibitor, in experimental glaucoma filtration surgery. Curr Eye Res. 2010;35(12):1090–8.
Hucker HB, Ahmad PM, Miller EA. Absorption, distribution and metabolism of dimethylsulfoxide in the rat, rabbit and guinea pig. J Pharmacol Exp Ther. 1966;154(1):176–84.
Edelhauser HF, Gallum AB, Van Horn DL, et al. Uptake and removal of dimethylsulfoxide in rabbit and human corneas during cryopreservation. Cryobiology. 1971;8:104–7.
Bourne WM, Shearer DR, Nelson LR. Human corneal endothelial tolerance to glycerol, dimethylsulfoxide, 1,2-propanediol, and 2,3-butanediol. Cryobiology. 1994;31(1):1–9.
Pult H, Korb DR, Blackie CA, Knop E, Marx E. About vital staining of the eye and eyelids. I. The anatomy, physiology, and pathology of the eyelid margins and the lacrimal puncta by E. Marx. 1924. Optom Vis Sci. 2010;87(10):718–24.
Tarrand JJ, Lasala PR, Han XY, Rolston KV, Kontoyiannis DP. Dimethyl sulfoxide enhances effectiveness of skin antiseptics and reduces contamination rates of blood cultures. J Clin Microbiol. 2012;50(5):1552–7.
Patil M. Pharmacology and clinical use of dimethyl sulfoxide (DMSO): a review. Int J Mol Vet Res. 2013:3(6).
Silverman C, Yoshizumi M. Ocular toxicity of experimental intravitreal DMSO. J Toxicol Cutan Ocul Toxicol. 1983;2(2–3):193–200.
Kiland J, Peterson J, Gabelt B, Kaufman P. Effect of DMSO and exchange volume on outflow resistance washout and response to pilocarpine during anterior chamber perfusion in monkeys. Curr Eye Res. 1997;16(12):1215–20.
Acknowledgments
The article processing charges for this publication were funded by ALC Therapeutics, LLC, Springhouse, PA, USA. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published.
Disclosures
J S. Pelletier, K. P. Stewart, K. Capriotti and Joseph A. Capriotti all have financial interest in ALC Therapeutics.
Compliance with ethics guidelines
Informed consent was obtained from the patient for publication of this case report.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Pelletier, J.S., Stewart, K.P., Capriotti, K. et al. Rosacea Blepharoconjunctivitis Treated with a Novel Preparation of Dilute Povidone Iodine and Dimethylsulfoxide: a Case Report and Review of the Literature. Ophthalmol Ther 4, 143–150 (2015). https://doi.org/10.1007/s40123-015-0040-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-015-0040-4