Abstract
Introduction
This study aimed to evaluate the prevalence of HIV-1 mutation V179D/E and the effect of V179D/E on the virological response to first-line efavirenz-based regimens among antiretroviral treatment (ART)-naïve patients.
Methods
An ambispective cohort study was conducted. All ART-naïve patients who underwent baseline genotypic resistance testing between January 2019 and November 2021 were included in the analysis of the prevalence of the V179D/E mutation. Then, patients with identified V179D/E received the efavirenz-based regimen or the protease inhibitor (PI)/integrase strand transfer inhibitor (INSTI)-based regimen. The virological and immunological outcomes at week 48 were compared between the two groups.
Results
HIV-1 mutation V179D/E was identified in 252 out of 2568 ART-naïve patients, with a prevalence of 9.8% in Shanghai, China. A total of 206 participants were included in the efficacy analysis. Forty-six patients with altered ART regimens or incomplete follow-up data were excluded from the analysis. The baseline characteristics were comparable between the efavirenz group (n = 109) and the PI/INSTI group (n = 97). At week 48, a total of 96 participants (88.1%) in the efavirenz group and 92 participants (94.8%) in the PI/INSTI group had a viral load lower than 50 copies/mL (chi-square test, p = 0.086). In both groups, a lower proportion of participants achieved virological suppression among participants with a baseline viral load of at least 100,000 copies/mL compared with those with lower than 100,000 copies/mL (66.7% vs. 96.1% in the efavirenz group, p < 0.001; 87.1% vs. 98.4% in the PI/INSTI group, p = 0.039). The median increase from baseline in the CD4 count at week 48 was significantly greater in the PI/INSTI group (192 cells/μL) than in the efavirenz group (154 cells/μL) (p = 0.029).
Conclusion
There is a high prevalence of V179D/E in ART-naïve patients with HIV-1 in Shanghai, China. The first-line efavirenz-based regimen may be not suitable for patients with HIV-1 mutation V179D/E, especially for those with a baseline viral load of at least 100,000 copies/mL. The study was registered at the Chinese Clinical Trial Registry (ChiCTR2000034787).
Similar content being viewed by others
The prevalence of the HIV-1 mutation V179D/E was 9.8% among ART-naïve patients in Shanghai, China. |
CRF01_AE and CRF55_01B were the most two common HIV-1 subtypes among the participants with HIV-1 V179D/E mutation. |
The efavirenz-based antiretroviral regimen showed poorer virological and immunological outcomes compared with the PI/INSTI-based regimen for ART-naïve patients with HIV-1 mutation V179D/E. |
The first-line efavirenz-based regimen is not recommended for patients with HIV-1 and V179D/E mutation, especially for those with a baseline viral load of at least 100,000 copies/mL. |
Introduction
HIV-1 is known to have a very high mutation rate and genetic diversity. HIV-1 drug resistance develops when the gene mutation occurs in the target region of anti-HIV-1 drugs, namely drug-resistance mutation (DRM). HIV-1 DRM not only leads to the failure of antiviral treatment but also to the further spread of drug-resistant strains. There are three main categories of HIV-1 drug resistance: acquired drug resistance (ADR), transmitted drug resistance (TDR), and pretreatment drug resistance (PDR) [1]. PDR is detected in antiretroviral treatment (ART)-naïve patients or ART-experienced patients who reported prior use of antiretroviral drugs reinitiating first-line ART. PDR is either TDR or ADR, or both. According to the World Health Organization (WHO)’s report on HIV drug resistance 2017, several countries estimated a prevalence of PDR greater than 10% in adults initiating ART, especially PDR to non-nucleoside reverse transcriptase inhibitors (NNRTI). A meta-analysis study showed that TDR had risen in China since 2012, and this rise was mainly driven by NNRTI resistance [2].
According to the Genotypic Resistance Test (GRT) Interpretation System of Stanford HIV Drug Resistance Database (HIVDB), the degree of susceptibility of HIV to antiretroviral drugs is divided into five levels: susceptibility (S), potential low-level resistance (P), low-level resistance (L), intermediate resistance (I), and high-level resistance (H) [3]. V179D is a polymorphic accessory NNRTI-selected mutation. It contributes low-level reductions in susceptibility to each of the NNRTIs. V179D has a weight of 1.0 in the Tibotec ETR genotypic susceptibility score. The combination of V179D and K103R acts synergistically to reduce nevirapine (NVP) and efavirenz (EFV) susceptibility. V179E is a non-polymorphic mutation occasionally selected by NVP and EFV. V179E appears similar to V179D in its effects on NNRTIs, with the same NNRTI resistance mutation scores as V179D. Studies conducted in China showed that the natural presence of the mutations V179D and V179E were found in HIV-1 strains CRF65_cpx and CRF55_01B, respectively [4, 5]. The trend of increasing V179D/E mutation in genotype CRF01_AE among men who have sex with men (MSM) population was also reported [6]. Our previous study found that the overall prevalence of PDR mutations in Shanghai, China was 17.4%, and V179D/E was the most frequent NNRTI-associated mutation, observed in 10.1% of patients [7].
NNRTIs-based ART regimens are recommended by China and WHO guidelines. In addition, EFV is the most widely used NNRTI drug. However, few studies have analyzed the impact of the a single V179D/E mutation which is associated with potential low-level resistance to NNRTIs on the virological response to a first-line EFV-containing regimen. This study aimed to investigate the prevalence of the V179D/E mutation in ART-naïve HIV-infected patients in Shanghai, China, and to determine whether the use of EFV-based ART regimens increased the risk of virologic failure in ART-naïve patients with the V179D/E mutation compared to non-NNRTI-based ART regimens. This study will help to clarify the clinical significance of HIV-1 V179D/E mutation.
Methods
Study Design and Participants
We conducted an ambispective cohort study in Shanghai Public Health Clinical Center (SPHCC), which is the only designated hospital providing the ART and long-term follow-up for HIV-1-infected patients in Shanghai, China. Data were collected prospectively from January 2020 onward and retrospectively before this date. All the ART-naïve patients who visited SPHCC during the period from January 2019 to November 2021 and had received baseline genotypic drug resistance testing were included in the analysis of the prevalence of V179D/E mutation, and then those identified with a single V179D/E mutation were enrolled in the following ART efficacy evaluation. Demographic, clinical, and laboratory data were collected at baseline and up to 48 weeks of ART. See Fig. 1.
Ethical approval was obtained from the Ethics Committee of SPHCC (2019-S044-02). This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. The informed consent was obtained from the prospectively enrolled patients, and waived for data collected retrospectively.
Identification of HIV-1 V179D/E Mutations
HIV-1 V179D/E mutations are NNRTI-associated DRMs. We performed viral RNA extraction, RT-PCR, and sequencing according to our previously established protocols [7]. The plasma samples were collected and preserved in a freezer at − 80 °C until analysis. Viral RNA was extracted from 140 μL plasma using the QIAmp Viral RNA Mini Kit (Qiagen, Germany) according to the manufacturer’s protocol. Then, the target fragment of 1316 bp in the pol gene spanning the reverse transcriptase and protease regions was amplified using a nested PCR. PrimeScript™ one-step RT-PCR ver. 2.0 (TakaRa, China) was used for the cDNA synthesis and first-round PCR operation. The nested PCR was performed with Ex Taq (TaKaRa, China). The PCR products were sent to BioSune Biotechnology Co. for sequencing (Applied Biosystems, 3730XL). The PCR protocol and primers used for PCR and sequencing were as described previously [7, 8]. HIV-1 DRMs and related resistance levels were determined on the basis of the Stanford University HIV Drug Resistance Database (HIVDB, https://hivdb.stanford.edu/hivdb/by-sequences/). After sequence analysis, HIV-1 V179D/E mutation frequencies were identified.
Evaluation of ART Regimens Among Participants with a Single HIV-1 V179D/E Mutation
Patients identified with a single V179D/E mutation were enrolled in the following ART efficacy assessment. They were assigned to receive the EFV-based ART regimen or the protease inhibitor (PI)/integrase inhibitor (INSTI)-based regimen according to the individuals’ wishes. In China, EFV and lopinavir/ritonavir (LPV/r) are available for free, while INSTI must be purchased at the patients’ own expense. All participants were to have study visits at baseline and every 12 weeks after starting ART, and physical examinations, laboratory tests, and patient compliance were assessed.
Study Endpoints
The main observation of this study was the efficacy of the EFV-based ART regimen compared with the PI/INSTI-based ART regimen in HIV-1-infected patients with a single V179D/E mutation. The primary endpoint was the proportion of participants with virological suppression at week 48 by US Food and Drug Administration (FDA) snapshot analysis. The secondary endpoint was the changes from baseline in CD4+ T cell counts at week 48 by FDA snapshot analysis. Virological suppression was defined as plasma HIV-1 RNA of less than 50 copies per milliliter (copies/mL) at week 48 after starting ART [9].
For participants in the prospective cohort, HIV-1 RNA was quantitatively measured at baseline and at weeks 12, 24, and 48, and CD4+ T cell counts were tested at baseline and every 12 weeks. For participants in the retrospective cohort, CD4+ T cell counts and HIV-1 RNA were measured for the first time, typically at week 12 and week 24, respectively, and then every 3–6 months and 6–12 months, respectively, in accordance with the routine follow-up management of HIV-1-infected patients.
HIV-1 RNA was detected and quantified by RT-PCR (COBAS® AmpliPrep/COBAS® TaqMan® HIV-1 Test, v2.0, ABI7500). CD4+ T cell counts were detected by flow cytometry using a BD FACS Canto II flow cytometry system (BD Multitest™ CD3/CD8/CD45/CD4, BD Biosciences).
Virological and immunological outcomes were compared between the two groups. In addition, subgroup analyses were performed between the two groups on the basis of baseline viral load and HIV-1 V179 mutation type.
HIV-1 Acquired DRMs in Participants with Baseline V179D/E Mutation
HIV-1 genotypic resistance testing was performed on participants at the time of virological failure during follow-up ART. Virological failure was defined as HIV-1 viral load of greater than 200 copies/mL after 24 weeks of ART. Participants who did not experience a viral load reduction of 1 log10 copies/mL from baseline after 12 weeks of ART were also tested for HIV-1 acquired DRMs.
Statistical Analysis
Data were analyzed using SPSS 22.0 (IBM Corp., Armonk, NY). The Kolmogorov–Smirnov test was used to assess the data for consistency. Continuous variables including age, CD4+ T cell counts, and HIV-RNA were described as median (interquartile range, IQR), and compared using Mann–Whitney U test. Categorical variables including gender and proportion of patients with virological suppression at week 48 were expressed as frequencies and percentages and compared using the chi-square (χ2) test or Fisher exact test. All tests were two tailed, and p < 0.05 is considered significant.
Results
Prevalence of HIV-1 V179D/E Mutation Among ART-Naïve Patients in Shanghai, China
The HIV-1 pol gene sequence was successfully amplified and analyzed from the plasma samples from 2568 ART-naïve HIV-1-infected patients. Among these patients, the single mutation of V179D/E was identified in 252 patients, with a prevalence of 9.8% (252/2568). See Fig. 1.
Demographic and Clinical Characteristics of Participants with V179D/E Mutation
Among the 252 treatment-naïve HIV-1-infected patients with the V179D/E mutation, 42 patients had incomplete follow-up data or missed follow-up, and 4 patients changed their treatment regimen within 6 months of treatment. Overall, a total of 206 participants were included in efficacy analysis. Among them, 109 participants were treated with the EFV-based regimen, and 97 participants were treated with the PI/INSTI-based regimen. See Fig. 1.
Demographic and clinical characteristics at baseline were well balanced between the two treatment groups (Table 1). The median age was 32 years; 95.1% of the participants were male. Participants with HIV-1 mutations V179D and V179E accounted for 44.2% and 55.8%, respectively. The median CD4+ T cell count was 266 (IQR 161–391) cells/μL. The median baseline viral load was 45,400 (IQR 18,000–126,000) copies/mL. The most two common HIV-1 subtypes were CRF01_AE (45.6%) and CRF55_01B (32.5%) among the participants with HIV-1 V179D/E mutation.
Virological Outcomes
At week 48, a total of 96 of 109 patients (88.1%) in the efavirenz group and 92 of 97 patients (94.8%) in the PI/INSTI group had a viral load of less than 50 copies/mL. See Fig. 2. Three patients in the PI/INSTI group had no virological data at week 48. If the three patients were not included in the analysis, fewer patients had viral suppression in the efavirenz group than in the PI/INSTI group (chi-square test, p = 0.013). If no virological data was regarded as treatment failure, there was no significant difference in the proportion of patients with a viral load of less than 50 copies/mL at week 48 between the two groups (chi-square test, p = 0.086). Figure 3 shows the proportion of patients with a viral load of less than 50 copies/mL over time.
Subgroup Analysis
Subgroup analyses were performed among the two treatment groups according to the baseline viral load (less than 100,000 copies/mL vs. at least 100,000 copies/mL) and the kind of HIV-1 V179 mutation (V179D vs. V179E). In both groups, a lower proportion of participants achieved virological suppression (with a viral load of less than 50 copies/mL at week 48) among participants with a baseline viral load at least 100,000 copies/mL compared with those with a baseline viral load of less than 100,000 copies/mL (66.7% vs. 96.1% in the EFV group, p < 0.001; 87.1% vs. 98.4% in the PI/INSTI group, p = 0.039). However, similar proportions of participants had a viral load of less than 50 copies/mL at week 48 in both the V179D and V179E subgroups (both between-group and within-group comparisons). Among participants with baseline viral load of at least 100,000 copies/mL, a slightly but not significantly lower proportion of participants achieved virological suppression in the EFV group (66.7%, 20/30) than in the PI/INSTI group (87.1%, 27/31; two cases with no virological data at week 48 were regarded as virological failure) (p = 0.073). See Fig. 4.
Subgroup analysis of the proportion of participants with a viral load of less than 50 copies per milliliter at week 48. There were three patients with missing baseline virological data in both EFV (efavirenz) and PI/INSTI (protease inhibitor/integrase strand transfer inhibitor) groups. VL viral load
Immunological Outcomes
The median change from baseline in the CD4+ T cell count at week 48 was significantly greater in the PI/INSTI group than in the efavirenz group, with an increase of 192 (IQR 120–264) cells/μL and 154 (IQR 84–247) cells/μL, respectively (p = 0.029). See Fig. 5.
HIV-1 Acquired DRMs Among Participants with Virological Failure at Week 48
HIV-1 genotypic resistance testing was performed on eight of the 15 participants who had not achieved virological suppression by week 48. Two patients in both groups had viral loads of 50–200 copies per milliliter at week 48.
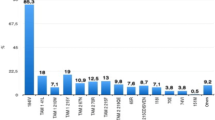
All eight participants who underwent HIV-1 resistance testing at the time of ADR at week 12 or 24 were from the EFV-based regimen group. Table 2 shows the characteristics of HIV-1 acquired resistance among these participants. Resistance to both NRTIs and NNRTIs was detected in all eight participants. M184V and V106M are the most common resistance mutations associated with NRTIs and NNRTIs, respectively.
Discussion
The widespread use of ART has resulted in rising levels of pretreatment HIV-1 drug resistance, especially in low-income and middle-income countries [10]. The prevalence of HIV-1 resistance to NNRTIs is much higher than other types of antiretroviral drugs, including PIs, NRTIs, and INSTIs [7, 11]. The study showed that individuals with PDR who initiated NNRTIs had an increased risk of virological failure and acquiring new resistance mutations compared to individuals without PDR [12]. V179D/E is an NNRTI-selected mutation that slightly reduces the virological response to first-line EFV-containing regimens. Our previous study found that potential low-level resistant to NNRTIs attributed to V179D/E was common in Shanghai, China [7]. More attention should be paid to the high prevalence of the V179D/E mutation among ART-naïve HIV-1-infected patients in China [13]. This study further investigated the prevalence of V179D/E in a larger population of HIV-1 infection and then assessed the impact of V179D/E on virological response to first-line NNRTI therapy. The results showed that the prevalence of the HIV-1 V179D/E mutation was 9.8% in Shanghai, China. For participants with the HIV-1 V179D/E mutation, the EFV-based regimen showed a poorer virologic outcome compared to the PI or INSTI-based regimen.
In our previous study, V179D/E was identified as the most common NNRTI mutation with a prevalence of 10.1% [7]. The current study found a similar prevalence of 9.8%. In addition, V179D and V179E have similar prevalence. In addition, we found that HIV-1 with the V179D/E mutation has its own unique subtype distribution. In addition to the main genotype CRF01_AE, CRF55_01B appears as the second most common genotype. In the general HIV-1 population, however, CRF55_01B accounts for only 1.9% of all subtypes [7]. Other studies have also reported the high prevalence of V179D/E in HIV-1 subtype CRF55_01B. One study conducted in Guangdong, China showed that V179E (98.77%) was the most frequent NNRTI DRM among CRF55_01B-infected individuals [14]. In another study, V179E was present in almost all of the included CRF55_01B-infected patients [5]. It is important to pay more attention to the trend of increasing prevalence of V179D/E among people with HIV-1 subtype CRF55_01B.
The risk of PDR contributing to treatment failure of NNRTI-based ART is associated with several factors, including the type and number of mutant codons, frequency of mutant variants in the individual’s viral quasispecies, mutant load, etc. [15]. If harboring low-frequency DRMs representing less than 1% of the viral quasispecies, naïve HIV-1-infected patients treated with first-line NNRTI-based treatment were not likely to experience virological failure [16]. The presence of the K103N mutant virus with a baseline load above 2000 copies/mL in ART-naïve individuals was associated with an increased risk of virologic failure in EFV-containing triple-drug regimens [17]. The failure threshold for different mutations may also depend on the potency of the ART regimen. V179D/E has a weight of 1.0 in the Tibotec ETR genotypic susceptibility score. A single V179D/E mutation results in potentially low-level HIV-1 resistance to the NNRTI. In this study, poorer virological response was observed in the EFV group than in the PI/INSTI group among participants with the HIV-1 V179D/E mutation, especially in those with a baseline viral load of at least 100,000 copies/mL. This suggests that a higher mutation viral load is a risk factor for treatment failure. However, in this study we did not quantify the frequency and load of mutant variants in individual viral quasispecies, which needs to be investigated further.
When V179D/E is combined with other DRM, treatment failure is more likely in patients with HIV-1 treated with a first-line NNRTI-based regimen. In this study, several additional DRMs were identified in participants with HIV-1 mutation V179D/E at the time of treatment failure, primarily the NRTI mutation M184V and the NNRTI mutation V106M. M184V causes high-level in vitro resistance to 3TC and FTC and low-level resistance to ABC. V106M is a non-polymorphic mutation that causes high-level resistance to NVP and EFV [3]. We also detected the mutation K103R/N in patients with treatment failure. The combination of V179D and K103R acts synergistically to reduce NVP and EFV susceptibility [3]. It is unknown whether these mutations were present at low frequencies in the pre-therapy viral quasispecies.
Limitations of this study include the non-randomized comparison between EFV- and PI/INSTI-based ART regimens and the relatively small sample size of the cohort. Antiretroviral treatment decisions were based on patient wishes and doctors’ suggestions. There may be some selection bias due to the non-randomized assignment of treatment regimens. A total of 46 patients were excluded from the efficacy analysis because of incomplete follow-up data or changes in treatment regimens. However, we did not describe the reasons for discontinuation of follow-up or changes in treatment regimens, including transfer to other sites, pursuit of simpler single-tablet medications, drug intolerance, etc, which may also have contributed to some bias. As discussed above, we did not detect the frequency and load of HIV-1 V179D/E mutant variants in individual viral quasispecies.
Conclusions
We found a high prevalence of the V179D/E mutation in ART-naïve patients with HIV-1 in Shanghai, China. The EFV-based ART regimens appeared to show poorer virological and immunological outcomes compared to the PI/INSTI-based regimens in ART-naïve patients with the HIV-1 V179D/E mutation. Therefore, the first-line EFV-based regimens may be not suitable for patients with HIV-1 and HIV-1 V179D/E mutation, especially for those with a baseline viral load of at least 100,000 copies/mL. These findings highlight the importance of assessing HIV-1 viral load and identifying HIV-1 resistance patterns at baseline in order to guide the appropriate choice of antiviral therapy.
References
WHO. Global action plan on HIV drug resistance 2017–2021. Geneva: World Health Organization; 2017. Licence: CC BY-NC-SA 3.0 IGO.
Zuo L, Liu K, Liu H, et al. Trend of HIV-1 drug resistance in China: a systematic review and meta-analysis of data accumulated over 17 years (2001–2017). EClin Med. 2020;18: 100238.
HIVDB Genotypic Resistance Test (GRT) Interpretation System. Updated October 2019. https://cms.hivdb.org/prod/downloads/release-notes/genotypic-resistance-test-interpretation-system-oct2019.pdf. Accessed 19 Aug 2022.
Liu Y, Zhang Y, Li H, et al. Natural presence of the V179D and K103R/V179D mutations associated with resistance to nonnucleoside reverse transcriptase inhibitors in HIV-1 CRF65_cpx strains. BMC Infect Dis. 2020;20(1):313.
Liu Y, Li H, Wang X, et al. Natural presence of V179E and rising prevalence of E138G in HIV-1 reverse transcriptase in CRF55_01B viruses. Infect Genet Evol. 2020;77: 104098.
Li X, Xue Y, Lin Y, et al. Evolutionary dynamics and complicated genetic transmission network patterns of HIV-1 CRF01_AE among MSM in Shanghai, China. Sci Rep. 2016;6:34729.
Wang Z, Zhang M, Zhang R, et al. Diversity of HIV-1 genotypes and high prevalence of pretreatment drug resistance in newly diagnosed HIV-infected patients in Shanghai, China. BMC Infect Dis. 2019;19(1):313.
Song YX, Xin RL, Li ZC, et al. Prevalence of transmitted drug resistance among HIV-1 treatment-naive patients in Beijing. Epidemiol Infect. 2018;146(3):339–44.
European AIDS Clinical Society Guidelines version 10.1. October 2020.
Su B, Zheng X, Liu Y, et al. Detection of pretreatment minority HIV-1 reverse transcriptase inhibitor-resistant variants by ultra-deep sequencing has a limited impact on virological outcomes. J Antimicrob Chemother. 2019;74(5):1408–16.
Yu F, Li Q, Wang L, et al. Drug resistance to HIV-1 integrase inhibitors among treatment-naive patients in Beijing, China. Pharmgenomics Pers Med. 2022;15:195–203.
Bertagnolio S, Hermans L, Jordan MR, et al. Clinical impact of pretreatment human immunodeficiency virus drug resistance in people initiating nonnucleoside reverse transcriptase inhibitor-containing antiretroviral therapy: a systematic review and meta-analysis. J Infect Dis. 2021;224(3):377–88.
Chen M, Zhu Q, Xing H, et al. The characteristics of pretreatment HIV-1 drug resistance in western Yunnan, China. Epidemiol Infect. 2020;148: e102.
Lan Y, Xin R, Cai W, et al. Characteristics of drug resistance in HIV-1 CRF55_01B from ART-experienced patients in Guangdong, China. J Antimicrob Chemother. 2020;75(7):1925–31.
Beck IA, Levine M, McGrath CJ, et al. Pre-treatment HIV-drug resistance associated with virologic outcome of first-line NNRTI-antiretroviral therapy: a cohort study in Kenya. EClin Med. 2020;18: 100239.
Nicot F, Sauné K, Raymond S, et al. Minority resistant HIV-1 variants and the response to first-line NNRTI therapy. J Clin Virol. 2015;62:20–4.
Goodman DD, Zhou Y, Margot NA, et al. Low level of the K103N HIV-1 above a threshold is associated with virological failure in treatment-naive individuals undergoing efavirenz-containing therapy. AIDS. 2011;25(3):325–33.
Acknowledgements
We thank all the participants of the study.
Funding
This work was funded by Shanghai Municipal Health Commission (201940225), Shanghai major projects on infectious diseases (shslczdzk01102), and Three-Year Action Plan for Strengthening Public Health System in Shanghai (GWV-10.1-XK02). The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Zhenyan Wang: conceptualization, methodology, writing - original draft, writing - review & editing, funding acquisition, software, formal analysis, investigation, visualization, writing - original draft, writing - review & editing. Min Zhang, Jiangrong Wang, Li Liu, Jun Chen, Renfang Zhang, Yinzhong Shen, Yang Tang, Tangkai Qi, Wei Song, Jianjun Sun, Shuibao Xu, Junyang Yang: investigation. Renfang Zhang, Hongzhou Lu: project administration, funding acquisition.
Disclosures
Zhenyan Wang, Min Zhang, Jiangrong Wang, Li Liu, Jun Chen, Renfang Zhang, Yinzhong Shen, Yang Tang, Tangkai Qi, Wei Song, Jianjun Sun, Shuibao Xu, Junyang Yang and Hongzhou Lu declare that they have no conflict of interest.
Compliance with Ethics Guidelines
This study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Ethical approval for this study was obtained from the Ethics Committee of Shanghai Public Health Clinical Center (2019-S044-02). The informed consent was obtained from the prospectively enrolled patients, and waived for data collected retrospectively. All data were de-identified and analyzed anonymously.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Wang, Z., Zhang, M., Wang, J. et al. Efficacy of Efavirenz-Based Regimen in Antiretroviral-Naïve Patients with HIV-1 V179D/E Mutations in Shanghai, China. Infect Dis Ther 12, 245–255 (2023). https://doi.org/10.1007/s40121-022-00723-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-022-00723-8