Abstract
Introduction
Cladribine administration has been approved for the treatment of relapsing–remitting multiple sclerosis (MS) in 2017; thus, data on cladribine in a real-world setting are still emerging.
Methods
We report on cladribine effectiveness, safety profile, and treatment response predictors in 243 patients with MS followed at eight tertiary MS centers. Study outcomes were: (1) No Evidence of Disease Activity-3 (NEDA-3) status and its components (absence of clinical relapses, MRI activity, and sustained disability worsening); (2) development of grade III/IV lymphopenia. The relationship between baseline features and the selected outcomes was tested via multivariate logistic models.
Results
Of the 243 subjects included in the study (66.5% female, age 34.2 ± 10 years, disease duration 6.6 ± 9.6 years), 64% showed NEDA-3 at median follow-up (22 months). Patients with higher number of previous treatments had lower probability to retain NEDA-3 [odds ratio (OR) 0.64, 95% confidence interval (CI) 0.41–0.98, p = 0.04] and were more prone to experience clinical relapses (OR 1.6, 95% CI 1–2.6, p = 0.04). The presence of active lesions at baseline was associated with follow-up magnetic resonance imaging (MRI) activity (OR 1.92, 95% CI 1.04–3.55, p = 0.04). Patients with higher rate of relapses in the year prior to cladribine start were at higher risk of developing sustained disability worsening (OR 2.95% CI 1–4.2, p = 0.04). Lymphopenia grade III/IV over the follow-up was associated with baseline lymphocyte count (OR 0.998, 95% CI 0.997–0.999, p = 0.01).
Conclusion
In this large cohort, we confirm previous data about cladribine effectiveness on disease activity and disability worsening and provide information on response predictors that might inform therapeutic choices.
Similar content being viewed by others
Cladribine administration has been approved for the treatment of relapsing–remitting multiple sclerosis (RRMS) in 2017 and data on cladribine in real-world setting are still emerging. |
Effectiveness, safety profile, and treatment response predictors in 243 MS patients followed at 8 tertiary MS centers in real-word practice. |
64% of patients included in the study showed No Evidence of Disease Activity (NEDA)-3 at median follow-up (22 months). Median time to NEDA-3 loss was 2.6 years. Patients with higher number of previous treatments had lower probability to retain NEDA-3 and were more prone to experience clinical relapses. III/IV grade lymphopenia over the follow-up was associated to baseline lymphocyte count (OR 0.998, 95% CI 0.997–0.999, p = 0.01). |
Our results advocate the use of higher efficacy disease-modifying therapy in early stages of the disease. |
Cladribine might be more effective when placed as an early choice along the treatment algorithm. |
Introduction
Cladribine (2-chlorodeoxyadenosine) is a purine nucleoside analog, approved for the treatment of relapsing–remitting multiple sclerosis (RRMS) in the European Union since 2017. Cladribine administration (two courses, 12 months apart) induces a deep, rapid, and long-lasting lymphocyte depletion, followed by an immune reconstitution, characterized by improved immune tolerance and reduced immune cell infiltration into the central nervous system [1]. This mechanism of action is responsible for cladribine sustained efficacy beyond the actual treatment period [2,3,4,5]. Indeed, in extension studies of the registration trial (CLARITY), over 70% of patients remained free from disability progression 5 years after baseline [5], with one-third of patients showing no evidence of disease activity (NEDA) up to 6 years [4]. In line with these findings, real-world data from the Italian MS national registry document show that over 50% of the patients treated with cladribine in the context of the randomized clinical trials (RCTs) did not relapse or experience disability progression after 5 years [6]. Finally, preliminary data from an exploratory single-center study suggest that cladribine might mitigate the risk of disability progression after 8 years [7]. Despite cladribine high and sustained efficacy, a percentage of patients show suboptimal response to the drug, with factors affecting treatment response still largely unknown. Post-hoc analyses from the CLARITY study reported consistent relapse rate reductions in all patients, regardless of baseline demographics and disease characteristics [8]. More recently, additional analyses suggested a better response in patients with high disease activity in the year prior to cladribine start [9], regardless of their pre-enrollment treatment status (naïve or previously treated) [10]. Data from real-world studies, including less disabled patients with RRMS with shorter disease duration and a larger variety of previous treatments compared with the RCTs, have suggested suboptimal disease control in cladribine-treated patients switching from natalizumab [11], and a higher risk of severe lymphopenia and subsequent herpes virus infections in patients previously treated with dimethyl fumarate [12]. As the MS therapeutic portfolio continues to expand, the identification and confirmation of potential risk factors of suboptimal response to specific disease-modifying therapies (DMTs) is of fundamental value. As, on the basis of CLARITY and extension trials, a full treatment course of oral cladribine does not require further therapy until at least 4 years after initiation of treatment [13], it is of utmost interest to identify factors that might predict treatment failure in shorter timeframes, in order to tailor therapeutic choices to individual needs. Given these premises, our study aims to explore cladribine effectiveness and safety profile and provide information on treatment response predictors, collecting data from eight tertiary MS centers in a retrospective observational setting.
Methods
Study Design
This was a multicenter, observational, post-marketing study. We retrospectively collected data of patients with RRMS who regularly attended eight tertiary MS outpatient clinics in Central and Southern Italy (A. Cardarelli Hospital, Naples; A.O.U. San Giovanni di Dio e Ruggi d'Aragona, Salerno; Department of Advanced Medical and Surgical Sciences, University of Campania “Luigi Vanvitelli,” Naples; Department of Neurosciences, Reproductive Science and Odontostomatology, Federico II University, Naples; IRCCS Neuromed, Pozzilli; Policlinico Tor Vergata, Rome; S. Andrea Hospital, Rome; San Paolo Hospital, Naples), and started treatment with cladribine after its approval by the Italian Medicine Agency (March 2019) or under a free-of-charge dispensation program. Administration of cladribine was performed according to the most recent summary of product characteristics [3.5 mg/kg body weight over 2 years, administered as one treatment course per year. Each treatment course consists of 2 treatment weeks, one at the beginning of the first month and one at the beginning of the second month of the respective treatment year. Each treatment week consists of 4 or 5 days on which a patient receives 10 mg or 20 mg (one or two tablets) as a single daily dose, depending on body weight]. Clinical and magnetic resonance imaging (MRI) data were collected by each MS center following the local medication monitoring plan and hospital guidelines. We considered data from patients who started cladribine as first treatment (naïves) as well as patients previously treated with other DMTs (either first-line therapies, e.g., dimethyl fumarate, glatiramer acetate, interferons, and teriflunomide, or second-line therapies, e.g., anti-CD20, daclizumab, fingolimod, mitoxantrone, and natalizumab). To analyze a population representative of clinical practice, patients who were originally enrolled in RCTs and continued treatment with cladribine after its approval were not included.
Data Collection
Data were retrospectively collected from January 2021 to December 2021 from each center clinical database, on a study-specific spreadsheet elaborated in order to harmonize data storage. For each patient, the following information was collected at cladribine start (henceforth defined as “baseline”): sex, date of birth, date of MS onset, date of MS diagnosis, Expanded Disability Status Scale (EDSS) score, number of relapses in the year preceding cladribine start, presence of gadolinium (Gd)-enhancing lesions at last MRI before treatment start (range 0–6 months), number of prior DMTs, last DMT, date and reason for its discontinuation, and date of first cladribine cycle. Follow-up data were collected from the baseline to the last available visit or until cladribine discontinuation. For each patient, we recorded the date of second cladribine cycle, number and dates of relapses, the occurrence and date of MRI activity, the presence and date of adverse events (AEs) during cladribine treatment, EDSS at each visit after cladribine start, lymphocyte count at months 3, 12, and 24 from cladribine start, and date and reason for cladribine discontinuation (if applicable). In December 2021, data were centrally reviewed and checked for consistency before analysis.
Outcome Measures
Effectiveness
As effectiveness outcomes, we considered NEDA-3 status and its components (absence of clinical relapses, MRI activity, and sustained disability worsening) [14]. A relapse was defined as any new neurologic symptom not associated with fever or infection lasting for at least 24 h and accompanied by new neurologic signs [15]. MRI activity was defined as the presence of new T2 hyperintense lesions compared with the baseline scan and/or Gd-enhancing lesions. Disability worsening was defined as 1.5-point increase (if baseline EDSS score was 0), 1.0-point increase (if baseline EDSS score was < 5.5), or 0.5-point increase (if baseline EDSS score was ≥ 5.5) confirmed 6 months apart and sustained until the last available visit [16]. We then identified the number of patients falling in the relapse-associated worsening (RAW) and progression independent of relapse activity (PIRA) category [17].
Only data of patients with a minimum 6-month persistence on cladribine were considered. The 6-month on-treatment persistence was decided on the basis of a phase 2 trial showing that improvements in MRI outcomes were evident at week 24, consistently with clinical outcomes [18, 19]. Indeed, differences in relapse rates between cladribine treatment groups and placebo demonstrated a positive trend as early as 4 weeks, with differences more marked and accompanied by non-overlapping confidence intervals after 24 weeks [18, 19]. For the same reasons, and to exclude rebound disease activity in patients switching from previous DMTs and misinterpretation related to regression to the mean effect [20], relapses and MRI activity occurring in the initial 6 months of treatment were not included in the effectiveness analysis.
Safety
As safety outcome, we considered the development of grade III/IV lymphopenia. Lymphopenia severity grades were defined according to current definition [21].
For descriptive purposes, AEs occurred under cladribine treatment were graded as mild (minimal or no treatment required and no interference with daily living activities), moderate (may require treatment and cause some interference with functioning), severe (systemic drug or other treatment required, interruption of daily living activities), or life threatening (immediate risk of death) [22].
Statistical Analysis
Statistical analyses were performed in SPSS 25.0, with a significance level α = 0.05. Descriptive data are provided for all variables included in the study. Continuous variables are summarized as mean values with standard deviations (SDs). Categorical variables are summarized as number (percentages). Between-group comparisons were tested with nonparametric Mann–Whitney U test for continuous variables and chi-square test for categorical variables. The relationship between baseline variables (demographic and clinical) and effectiveness outcomes was tested via multivariate logistic models. We chose to perform a multivariate regression analysis, entering in the model a set of predictors and assessing their individual contribution to the overall prediction rather than a preliminary univariate model. The application of a univariate model to the prediction of the outcome(s) would not reflect the real-world setting were the effects of/interaction between multiple clinical and demographic factors (rather than the effect of a single factor observed in isolation) driving the observed outcome(s).
Outcomes of interest were NEDA-3 at median follow-up (primary outcome), occurrence of relapses over the follow-up, occurrence of MRI activity over the follow-up, sustained disability worsening at last available follow-up. All models accounted for the following covariates: sex, age at cladribine start, disease duration, number of treatments before cladribine, relapses in the year before cladribine start, presence of MRI active lesions at baseline, switch or naïve status. The model predicting sustained disability worsening also accounted for basal EDSS. We performed a logistic multivariate regression predicting NEDA-3 at a fixed follow-up (namely the median follow-up of 22 months), thus limiting the issue related to the follow-up variability within our cohort. For consistency, the same analysis was performed for NEDA subcomponents (relapse, MRI activity, and disability worsening). Moreover, predictive variables of time to NEDA-3 loss were also estimated with a Cox regression analysis; median survival time to NEDA-3 loss and its components were estimated by Kaplan Meier survival analysis. MRI activity occurring within 1 month from a clinical relapse was considered associated with the clinical relapse and therefore considered in the model predicting occurrence of relapses over the follow-up rather than in the model predicting MRI activity over the follow-up. The choice to analyze clinical activity and MRI activity separately was sustained by their different biological and prognostic meaning. Indeed, the presence of minimal evidence of MRI activity is prognostically favorable [23], while clinical activity has a recognized impact on disability accrual [24].
The relationship between baseline variables and safety outcomes (grade III/IV lymphopenia) was tested via multivariate logistic model, accounting for sex, age at cladribine start, disease duration, number of treatments before cladribine, relapses in the year before cladribine start, basal lymphocyte count, and switch or naïve status.
Post-hoc analyses were run to explore the impact of exposure to different DMTs on our primary outcomes (Kaplan–Meier curves and log-rank comparisons for time to NEDA-3 loss and logistic regression for grade III/IV lymphopenia). Prior DMT exposure was reclassified as “naïve/first-line injectables/first-line oral/fingolimod/natalizumab” as well as “naïve/first line/second line.” Only treatments with a frequency higher than 1% in the study population were included in these analyses. In the time-to-event analysis, for patients experiencing both clinical and MRI activity over the follow-up, the event that occurred first chronologically was considered for the time to NEDA-3 loss.
Ethics Statement
The present study was conducted in accordance with specific national laws and the ethical standards of the 1964 Declaration of Helsinki and its later amendments. Given its retrospective design, this study did not interfere in the care received by patients. In addition, specific ethical approval was not required owing to the retrospective design, and since all clinical assessments were part of the clinical practice in a university- or hospital-based specialized center setting. However, as per Italian regulations [25], the principal investigator of each site notified the local ethic committee about this retrospective study. Patients provided their informed consent to collect data for clinical purposes.
Results
Study Population
A total of 243 subjects were included in the study (66.5% female, mean age 34.2 ± 10 years, mean disease duration 6.6 ± 9.6 years). Of these, 71 (29.3%) were treatment naïve, while 172 (70.7%) were switching from previous therapies (72.6% from first-line and 27.3% from second-line treatments) (Fig. 1). Reasons for switch were inefficacy (124 patients, 72%), safety concerns (31 patients, 18.1%), or other reasons (pregnancy planning, personal choice of the patient, low compliance) (16 patients, 9.3%). Naïve patients were significantly younger and showed shorter disease duration than patients switching from other therapies (32.86 ± 10.5 versus 35.7 ± 9.6 years p = 0.02 and 1.54 ± 3.0 versus 8.74 ± 6.8 years p < 0.0001, respectively). The mean wash-out period between previous treatment and cladribine start was 3.7 months. The relapse rate in the year prior to cladribine start was 0.69, and 56.7% of the patients had active lesions prior to cladribine start. Naïve patients and patients switching from other therapies did not differ for Annualized Relapse Rate (ARR) in the year prior to cladribine start, presence of active lesions, or EDSS at baseline. The mean follow-up from cladribine start was 22 ± 10 months (median 22 months, range 8–45 months). Eighty-four percent of the enrolled patients had completed two cycles of treatments and 21% were followed up for 2 years or more. Demographics and baseline clinical data of the study population are shown in Table 1.
Treatment frequencies before cladribine start. Pie chart illustrating last treatment before cladribine start (expressed as treated patients/total population × 100). DAC: daclizumab; DMF: dimethyl fumarate; FTY: fingolimod; GA: glatiramer acetate; IFNs: interferons; NTZ: natalizumab; MTX: mitoxantrone; TERI: teriflunomide
Effectiveness
No Evidence of Disease Activity-3
Sixty-four percent of patients showed no evidence of disease activity (NEDA-3) at median follow-up (22 months). Patients with higher number of previous treatments had lower probability to retain NEDA-3 (OR 0.64, 95% CI 0.41–0.98, p = 0.04). Descriptive data and results of the logistic regression investigating the relationship between demographic/clinical features and NEDA-3 are reported in Table 2. Higher number of previous treatments was also associated with time to NEDA-3 loss (p = 0.03). On the other hand, time to NEDA-3 loss did not differ across DMTs exposure classes (either first-line injectables/first-line oral/fingolimod/natalizumab or first line/second line) (data not shown). Median time to NEDA-3 loss was 2.6 years. Figure 2 shows median survival time to NEDA-3 loss and its subcomponents (relapse, MRI activity, disability worsening). Respectively median survival time to first relapse was 3.2 years, median survival time to MRI activity was 2.6 years, and median survival time to disability worsening was 2.9 years.
Median survival time to NEDA-3 loss and its subcomponents. A Median survival time to NEDA-3 loss; B median survival time to first relapse; C median survival time to MRI activity (new T2 lesions or/and gadolinium enhancing lesions); D median survival time to disability worsening. NEDA-3, no evidence of disease activity; MRI magnetic resonance imaging
Relapses
After cladribine start, clinical relapses were registered in 28 patients (28 events, 72.7% in patients switching from other therapies). Excluding events occurred during the first 6 months of treatment (10 clinical relapses), 18 relapses were included in the analysis. Higher number of previous treatments was the only baseline feature associated with higher probability to experience clinical relapses (OR 1.6, 95% CI 1–2.6, p = 0.04, respectively). Descriptive data and results of the logistic regression investigating the relationship between demographic/clinical features and relapses over the follow-up are reported in Table 3.
Magnetic Resonance Imaging Activity
Evidence of MRI activity was noted in 55 patients (55 events, 76.9% percent in patients switching from other therapies). Excluding events occurred in association to clinical relapses (n = 15) and or in the first 6 months of therapy (n = 10), 30 events were included in the analysis. Among baseline features, only the presence of active lesions detected at baseline MRI was associated to follow-up MRI activity (OR 1.92, 95% CI 1.04–3.55, p = 0.04). Descriptive data and results of the logistic regression investigating the relationship between demographic/clinical features and MRI activity over the follow-up are reported in Table 4.
Sustained Disability Worsening
Considering the last available follow-up, sustained disability worsening occurred in 31 patients (12.7%). Among sustained disability worsening events, 3 could be classified as RAW and 28 as PIRA. Given the low number of RAW events, we did not have the power to perform a separate analysis in this group. Indeed, patients with a higher rate of relapses in the year prior to cladribine start were at higher risk of developing sustained disability worsening at follow-up (OR 2.95%, 95%CI 1–4.2, p = 0.04); with a trend identified also for higher EDSS at baseline (OR 0.55, 95%CI 0.29–1.0, p = 0.07). Descriptive data and results of the logistic regression investigating the relationship between demographic/clinical features and sustained disability worsening at last available follow-up are reported in Table 5.
Switch to Other Therapies
During the follow-up, 11 patients (4%) switched from cladribine to other treatments (ocrelizumab, natalizumab, siponimod) following disease activity (n = 8) or disability worsening (n = 3). Three patients switched after one cycle (mean time to switch 10 ± 1 months) and eight patients after two cycles (mean time to switch 34 ± 4 months).
Safety
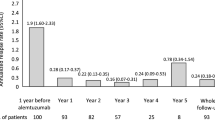
Lymphocyte levels were available in 220 patients at baseline, 183 patients at 3 months, 151 patients at year 1, and 79 patients at year 2. Mean lymphocyte count was 1907.6 ± 825.6 at baseline, 1025.03 ± 438.5 after 3 months, 1159.62 ± 446.1 after 1 year, and 1060.2 ± 408.8 after 2 years from treatment start (Fig. 3). Eleven patients had their second cladribine cycle delayed (mean delay time 2.2 ± 1.6 months) following a reduced lymphocyte count at year 1 (mean 651.82 ± 115.57).
Three months after treatment start, we observed 2 cases of grade IV lymphopenia, 17 cases of grade III lymphopenia, 50 cases of grade II lymphopenia, and 24 cases of grade I lymphopenia. Eighty-four percent of patients showing grade III/IV lymphopenia had been previously exposed to DMTs (seven fingolimod, six dimethyl fumarate, three glatiramer acetate, and one teriflunomide). Lymphopenia grade III/IV over the follow-up was associated with baseline lymphocyte count (OR 0.998, 95% CI 0.997–0.999, p = 0.01). Descriptive data and results of the logistic regression investigating the relationship between demographic/clinical features and grade III/IV lymphopenia are reported in Table 6.
Grade III/IV lymphopenia was not influenced by DMTs exposure classes (either first-line injectables/first-line oral/fingolimod/natalizumab or first line/second line) (data not shown).
In the whole cohort we registered 12 mild AEs in 11 patients, including the 2 aforementioned grade IV lymphopenia, 1 transaminase increase, 3 infections (herpes zoster, herpes simplex, vaginal candidiasis), 3 dermatitis, and 1 alopecia.
Discussion
Real-world evidence from diverse population is extremely valuable in determining drug effectiveness and safety outside of the selected conditions of RCTs. Here, analyzing 243 patients treated with cladribine over a mean period of 22 months, we confirm previous data about cladribine effectiveness on disease activity and disability worsening, and provide information on response predictors that might inform therapeutic choices. Indeed, the complementary role of real-world evidence and RCTs findings in guiding decision making, widely acknowledged in clinical practice, has been recently recognized also by regulatory authorities [26].
Here, in comparison with the population analyzed in the phase III study CLARITY and its extension, we present data on younger (34 versus 38 years) and less disabled (mean EDSS 1.9 versus 2.8) patients, often starting cladribine after previous DMTs (71% versus 26%) and exposed to a wide variety of previous treatments, including, but not limited to, injectable drugs. Different from other recent real-world data [6, 27], patients included in the present study were all treated with the licensed dose of 3.5 mg/kg and, for the vast majority (84%), completed two courses of cladribine therapy.
Regarding cladribine effectiveness, we found high rates of NEDA-3 after 22 months of cladribine treatment, substantially overlapping with previous data from CLARITY post-hoc analysis [28] and real-world evidence on NEDA-3 at 2 years after treatment with bioequivalent intravenous cladribine administered in a single center before 2010 [29] (respectively 64%, 67%, and 65% of patients free from disease activity and progression). The only factor affecting the likelihood of retaining NEDA and the risk to experience new relapses was the number of prior treatments, in agreement with previous data analyzing effectiveness of intravenous cladribine and alemtuzumab [29], which advocate the use of higher efficacy disease-modifying therapy in early stages. This finding should be interpreted with caution, since demographical and clinical characteristics of naïve and previously treated patients were different and matching among the two groups was not performed. However, NEDA-3 was not affected by previous treatment exposure (i.e., patients treated with first- versus second-line DMTs did not show any difference in time to NEDA in comparison with naïve patients). Indeed, consistent treatment benefit in patients with and without prior exposure to DMTs had been already reported in post-hoc analyses of the CLARITY study [8, 10]. Patients showing Gd-enhancing lesions at baseline had higher risk to develop MRI activity over the follow-up. This finding is not surprising, given that Gd-enhancing lesions are a good predictor for short-term MRI activity [20]. In line with a post-hoc analysis exploring disability outcomes at 5 years from CLARITY baseline [4], about 12% of patients in our cohort showed EDSS worsening over the follow-up. Risk of sustained worsening was higher in patients with higher relapse rate in the year before cladribine start. Although this finding might seem counterintuitive, as previous data support an amplified beneficial effect of cladribine tablets in highly active patients [9, 30], the definition of “high activity” usually requires the occurrence of two relapses in the year prior to treatment start, and the relapse rate in our cohort was far from this cutoff (0.7 in the overall population, 0.9 in patients experiencing progression). Thus, our results are better interpreted in the light of a recent study that, analyzing more than 27,000 patients with ≤ 15.7 years of follow-up, has confirmed the major contribution of relapses to the accumulation of disability over the course of MS [31].
Lymphocyte counts in our cohort followed the kinetics known from previous RCTs, with lymphopenia peaking at month 3 [18, 32]. Incidence of grade IV lymphopenia was similar to the one reported in clinical trials (< 1%), while the incidence of grade III lymphopenia was even lower (7% versus 25%) [32].
The risk of developing grade III/IV lymphopenia was higher in patients with lower lymphocyte count at baseline, in line with previous reports on patients treated with interferon, fingolimod, and dimethyl-fumarate [33,34,35]. These data might be useful to stratify patients according to their risk of developing lymphopenia, guiding personalized therapeutic choices. However, although an integrated analysis of cladribine safety from phase III clinical trials and subsequent long-term observational registry has reported increased frequency of infections during periods of grade III/IV lymphopenia[32], none of the infections described in our population occurred in the context of severe lymphopenia. Indeed, risk stratification for opportunistic infections in MS patients should take into account an array of individual demographic and clinical features [36], and risk mitigation can be achieved through the implementation of an adequate screening and prophylaxis protocol [37].
Despite analyzing a cohort substantially comparable in terms of sample size and demographic- and disease-related features to the one recruited from Germany tertiary centers [12], we did not identify any prior exposure to specific DMTs as risk factor for suboptimal response to cladribine.
Our work is not free from limitations. Indeed, the retrospective design of the study implies the possibility that certain variables, potentially influencing the outcome, may not have been recorded. However, a study-specific spreadsheet for data collection was set up before study initiation and provided to each center in order to collect a minimal common set of fundamental information for all enrolled patients. Additionally, the relatively small sample size and short follow-up might have limited our ability to detect differences within our population, and further real-world studies on larger cohorts with longer follow-up will be needed to address these topics.
Conclusions
Overall, in this real-word study we confirmed previous data on effectiveness and safeness of cladribine in RRMS. Moreover, we identified better outcomes in patients exposed to a lower number of previous DMTs, suggesting that cladribine might be more effective when placed as an early treatment along the treatment algorithm. Results from ongoing trials are awaited to support the long-term benefit of induction versus escalation therapy [38, 39].
References
Jacobs BM, Ammoscato F, Giovannoni G, Baker D, Schmierer K. Cladribine: mechanisms and mysteries in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2018;89:1266–71.
Giovannoni G, Soelberg Sorensen P, Cook S, Rammohan K, Rieckmann P, Comi G, et al. Safety and efficacy of cladribine tablets in patients with relapsing–remitting multiple sclerosis: results from the randomized extension trial of the CLARITY study. Mult Scler J. 2018;24:1594–604.
De Stefano N, Sormani MP, Giovannoni G, Rammohan K, Leist T, Coyle PK, et al. Analysis of frequency and severity of relapses in multiple sclerosis patients treated with cladribine tablets or placebo: the CLARITY and CLARITY Extension studies. Mult Scler J. 2022;28:111–20.
Giovannoni G, Singer BA, Issard D, Jack D, Vermersch P. Durability of no evidence of disease activity-3 (NEDA-3) in patients receiving cladribine tablets: the CLARITY extension study. Mult Scler J. 2021. https://doi.org/10.1177/13524585211049392.
Giovannoni G, Comi G, Rammohan K, Rieckmann P, Dangond F, Keller B, et al. Long-term disease stability assessed by the expanded disability status scale in patients treated with cladribine tablets 3.5 mg/kg for relapsing multiple sclerosis: an exploratory post hoc analysis of the CLARITY and CLARITY extension studies. Adv Ther. 2021;38:4975–85.
Patti F, Visconti A, Capacchione A, Roy S, Trojano M, CLARINET-MS Study Group. Long-term effectiveness in patients previously treated with cladribine tablets: a real-world analysis of the Italian multiple sclerosis registry (CLARINET-MS). Ther Adv Neurol Disord. 2020;13:1756286420922685.
Moccia M, Lanzillo R, Petruzzo M, Nozzolillo A, De Angelis M, Carotenuto A, et al. Single-center 8-years clinical follow-up of cladribine-treated patients from phase 2 and 3 trials. Front Neurol. 2020;11:489.
Rammohan K, Giovannoni G, Comi G, Cook S, Rieckmann P, Soelberg Sørensen P, et al. Cladribine tablets for relapsing-remitting multiple sclerosis: Efficacy across patient subgroups from the phase III CLARITY study. Mult Scler Relat Disord. 2012;1:49–54.
Giovannoni G, Soelberg Sorensen P, Cook S, Rammohan KW, Rieckmann P, Comi G, et al. Efficacy of Cladribine Tablets in high disease activity subgroups of patients with relapsing multiple sclerosis: a post hoc analysis of the CLARITY study. Mult Scler. 2019;25:819–27.
Vermersch P, Galazka A, Dangond F, Damian D, Wong SL, Jack D, et al. Efficacy of cladribine tablets in high disease activity patients with relapsing multiple sclerosis: post hoc analysis of subgroups with and without prior disease-modifying drug treatment. Curr Med Res Opin. 2021;37:459–64.
Zanghì A, Gallo A, Avolio C, Capuano R, Lucchini M, Petracca M, et al. Exit strategies in natalizumab-treated RRMS at high risk of progressive multifocal leukoencephalopathy: a multicentre comparison study. Neurotherapeutics. 2021;18:1166–74.
Pfeuffer S, Rolfes L, Hackert J, Kleinschnitz K, Ruck T, Wiendl H, et al. Effectiveness and safety of cladribine in MS: Real-world experience from two tertiary centres. Mult Scler. 2022;28:257–68.
European Medicines Agency. Mavenclad (cladribine tablets), summary of product characteristics. 2021. https://www.ema.europa.eu/en/documents/product-information/mavenclad-epar-product-information_en.pdf. Accessed 21 Mar 2022.
Giovannoni G, Turner B, Gnanapavan S, Offiah C, Schmierer K, Marta M. Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Mult Scler Relat Disord. 2015;4:329–33.
Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–73.
Rio J, Nos C, Tintorè M, Tèllez N, Galan I, Pelayo R, et al. Defining the response to interferon-beta in relapsing-remitting multiple sclerosis patients. Ann Neurol. 2006;59:344–52.
Kappos L, Wolinsky JS, Giovannoni G, Arnold DL, Wang Q, Bernasconi C, et al. Contribution of relapse-independent progression vs relapse-associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77:1132–40.
Giovannoni G, Comi G, Cook S, Rammohan K, Rieckmann P, Soelberg Sørensen P, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362:416–26.
Comi G, Cook SD, Giovannoni G, Rammohan K, Rieckmann P, Sørensen PS, et al. MRI outcomes with cladribine tablets for multiple sclerosis in the CLARITY study. J Neurol. 2013;260:1136–46.
Stellmann J-P, Stürner KH, Young KL, Siemonsen S, Friede T, Heesen C. Regression to the mean and predictors of MRI disease activity in RRMS placebo cohorts–is there a place for baseline-to-treatment studies in MS? PLoS ONE. 2015;10: e0116559.
Common Terminology Criteria for Adverse Events (CTCAE). Protocol Development. CTEP. 2022. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm. Accessed 21 Mar 2022.
ICH GCP-Essential documents for the conduct of a clinical trial-ICH GCP. 2022. https://ichgcp.net/8-essential-documents-for-the-conduct-of-a-clinical-trial. Accessed 21 Mar 2022.
Prosperini L, Mancinelli C, Haggiag S, Cordioli C, De Giglio L, De Rossi N, et al. Minimal evidence of disease activity (MEDA) in relapsing-remitting multiple sclerosis. J Neurol Neurosurg Psychiatry. 2020;91(3):271–7.
Prosperini L, Ruggieri S, Haggiag S, Tortorella C, Pozzilli C, Gasperini C. Prognostic accuracy of NEDA-3 in long-term outcomes of multiple sclerosis. Neurol Neuroimmunol Neuroinflammation. 2021;8(6): e1059.
AIFA. Agenzia Italiana del Farmaco (AIFA). Determinazione dell’Agenzia Italiana del Farmaco, 20 Marzo 2008. Gazzetta Ufficiale della Repubblica Italiana. 2008;76:67–74.
Real-World Evidence. FDA. 2022. https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence. Accessed 21 Mar 2022.
Lizak N, Hodgkinson S, Butler E, Lechner-Scott J, Slee M, McCombe PA, et al. Real-world effectiveness of cladribine for Australian patients with multiple sclerosis: an MSBase registry substudy. Mult Scler. 2021;27:465.
Giovannoni G, Cook S, Rammohan K, Rieckmann P, Sørensen PS, Vermersch P, et al. Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: a post-hoc and subgroup analysis. Lancet Neurol. 2011;10:329–37.
Bose G, Rush C, Atkins HL, Freedman MS. A real-world single-centre analysis of alemtuzumab and cladribine for multiple sclerosis. Mult Scler Relat Disord. 2021;52: 102945.
Signori A, Saccà F, Lanzillo R, Maniscalco GT, Signoriello E, Repice AM, et al. Cladribine vs other drugs in MS. Neurol Neuroimmunol Neuroinflamm. 2020;7: e878.
Lublin FD, Häring DA, Ganjgahi H, Ocampo A, Hatami F, Čuklina J, et al. How patients with multiple sclerosis acquire disability. Brain. 2022. https://doi.org/10.1093/brain/awac016.
Cook S, Leist T, Comi G, Montalban X, Giovannoni G, Nolting A, et al. Safety of cladribine tablets in the treatment of patients with multiple sclerosis: an integrated analysis. Mult Scler Relat Disord. 2019;29:157–67.
Lim ZW, Elwood E, Naveed H, Galea I. Lymphopenia in treatment-naive relapsing multiple sclerosis: table. Neurol Neuroimmunol Neuroinflamm. 2016;3: e275.
Morales FS, Koralnik IJ, Gautam S, Samaan S, Sloane JA. Risk factors for lymphopenia in patients with relapsing–remitting multiple sclerosis treated with dimethyl fumarate. J Neurol. 2020;267:125–31.
Warnke C, Dehmel T, Ramanujam R, Holmen C, Nordin N, Wolfram K, et al. Initial lymphocyte count and low BMI may affect fingolimod-induced lymphopenia. Neurology. 2014;83:2153–7.
Fox EJ, Buckle GJ, Singer B, Singh V, Boster A. Lymphopenia and DMTs for relapsing forms of MS: considerations for the treating neurologist. Neurol Clin Pract. 2019;9:53.
Zappulo E, Buonomo AR, Moccia M, Pinchera B, Villari R, Petracca M, et al. Impact of an anti-infective screening and monitoring protocol together with infectious disease consultation in preventing infective adverse events in patients treated with anti-CD20/CD52 agents for multiple sclerosis. Mult Scler Relat Disord. 2022;63: 103814.
Traditional Versus Early Aggressive Therapy for Multiple Sclerosis Trial-Full Text View-ClinicalTrials.gov. 2022. https://clinicaltrials.gov/ct2/show/NCT03500328. Accessed 21 March 2022.
Determining the Effectiveness of Early Intensive Versus Escalation Approaches for RRMS-Full Text View-ClinicalTrials.gov. 2022. https://clinicaltrials.gov/ct2/show/NCT03535298. Accessed 21 Mar 2022.
Acknowledgements
The authors thank the subjects who participated in this real word study and support staff.
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Author Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by all authors. Statistical analysis was performed by Maria Petracca and Elisabetta Signoriello. The first draft of the manuscript was written by Maria Petracca, Serena Ruggieri and Elisabetta Signoriello and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Maria Petracca has received research grants from FISM, speaking/consulting honoraria from HEALTH&LIFE Srl and Biogen; Serena Ruggieri has received honoraria from Biogen, Merck Serono, Novartis, Roche, Bristol Myers Squibb, Sanofi Genzyme, Viatris for consulting services, speaking and/or travel support; Elena Barbuti has nothing to disclose; Antonio Ianniello has received consultancy honoraria from Janssen; Roberta Fantozzi received speaker honoraria and/or consultancy from Biogen, Merck, Novartis, Roche; Giorgia Teresa Maniscalco has received personal compensations from Serono, Biogen, Novartis, Roche and TEVA for public speaking and advisory boards; Vincenzo Andreone has nothing to disclose; Doriana Landi has received travel funding from Biogen, Merck Serono, Sanofi- Genzyme, Teva, speaking or consultations fees from Viatris, Sanofi-Genzyme, Merck Serono, Teva, Biogen, Roche; Girolama Alessandra Marfia has received travel funding, speaking or consultation fees from Viatris, Almirall, Bayer-Schering, Biogen, Genzyme, Merck Serono, Novartis, Teva, Sanofi-Genzyme. Maria Di Gregorio has nothing to disclose; Rosa Iodice has received speaker honoraria and/or consultancy from Biogen, Teva, Genzyme, Merck, Almirall, Roche; Leonardo Sinisi has received advisory board honoraria and/ or congress grants from Almirall, Biogen, Merck, Novartis, Roche, Viatris; Elisabetta Maida has nothing to disclose; Rosanna Missione has nothing to disclose; Cinzia Coppola has nothing to disclose; Simona Bonavita has received speaker honoraria and/or advisory board fee, travel grants from Novartis, Teva, Roche, Sanofi- Genzyme, Biogen, Merck-Serono, Mylan; Giovanna Borriello has received fees for advisory boards, consulting and travel adjustment from Almirall, Biogen, Merck, Novartis, Roche, Sanofi-Genzyme, Teva; Diego Centonze is an advisory board member of Almirall, Bayer Schering, Biogen, GW Pharmaceutical, Merck Serono, Novartis, Roche, Sanofi- Genzyme, and Teva and received honoraria for speaking or consultation fee from Almirall, Bayer Schering, Biogen, GW Pharmaceuticals, Merck Serono, Novartis, Roche, Sanofi- Genzyme, and Teva; he is also the principal investigator in clinical trials for Bayer Schering, Biogen, Merck Serono, Novartis, Roche, and Sanofi-Genzyme; Giacomo Lus has received speaker honorariaand/or consultancy from Biogen, Teva, Genzyme, Merck, Novartis, Almirall, Roche; Carlo Pozzilli has served on scientific advisory boards for Novartis, Merck, Biogen, Sanofi, Genzyme, Teva, and Actelion; received funding for travel and speaker honoraria from Biogen, Teva, Sanofi Genzyme, Actelion, and Novartis; received research support from Biogen, Teva, Novartis, and Genzyme; Elisabetta Signoriello has received speaker honoraria and/or consultancy from Biogen, Teva, Genzyme, Merck, Novartis, Almirall, Roche.
Compliance with Ethics Guidelines
The present study was conducted in accordance with specific national laws and the ethical standards of the 1964 Declaration of Helsinki and its later amendments. Given its retrospective design, this study did not interfere in the care received by patients. In addition, specific ethical approval was not required due to the retrospective design and since all clinical assessments were part of the clinical practice in a university- or hospital- based specialized center setting. However, as per Italian regulations [22], the principal investigator of each site notified the local ethic committee about this retrospective study. Patients provided their informed consent to collect data for clinical purposes.
Data Availability
Anonymized data not published within this article will be made available by request from any qualified investigator.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Petracca, M., Ruggieri, S., Barbuti, E. et al. Predictors of Cladribine Effectiveness and Safety in Multiple Sclerosis: A Real-World, Multicenter, 2-Year Follow-Up Study. Neurol Ther 11, 1193–1208 (2022). https://doi.org/10.1007/s40120-022-00364-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40120-022-00364-6